Camber Spine, a leading innovator in spine and medical technologies has announced a robust calendar of clinical education and product portfolio expansions in 2022.
Camber Spine notes plans for the year include four spine surgical training labs focused on the Oblique Lateral Interbody Fusion Surgery (OLIF) technique. The labs, part of the Camber Education Program, are designed to give surgeons an opportunity to further their experience accessing the anterior spine (L2-S1) through an oblique approach. The program faculty will use didactic sessions, hands-on cadaveric training and case review to provide a full-day session to benefit surgeon attendees. Additional training labs on will be offered throughout the year on ALIF, LLIF, PLIF and TLIF techniques.
Camber Spine will also be offering unique OLIF training opportunities via its one-on-one Clinical Preceptor Training program with Dr. John I. Williams, Spine Surgeon, ORTHO Northeast, Fort Wayne, IN.
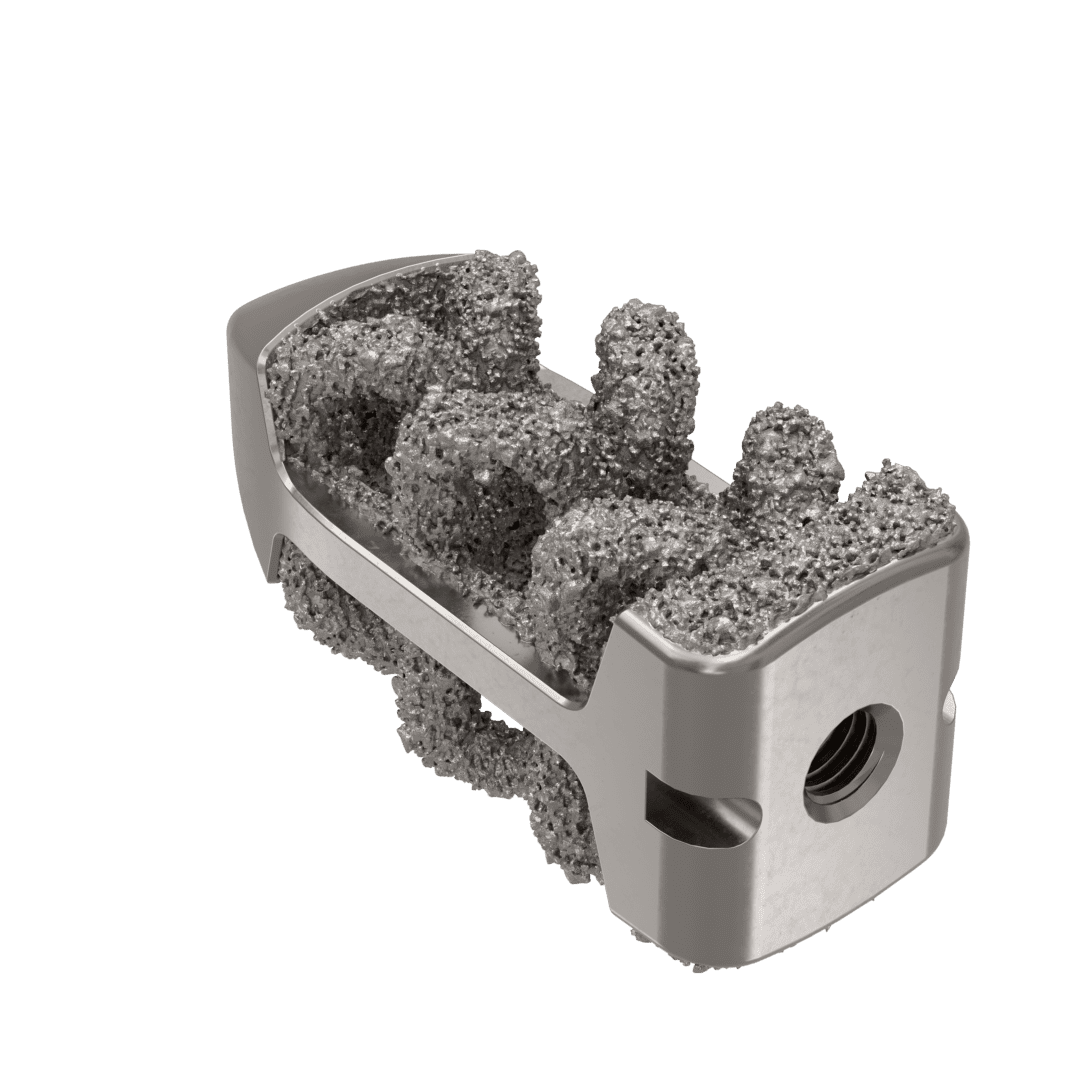
In addition, Camber’s product pipeline is scheduled to produce a number of key product launches throughout 2022, starting with innovative navigation and MIS instruments to be announced later in Q1 and products to compliment a complete posterior approach, also in Q1. Camber will also continue to expand its award-winning Spira and Enza technology platforms in the first three quarters of this year, with a series of product launches, including a full compliment of biologic options.
Camber Spine will be exhibiting and presenting its products (including SPIRA-T, SPIRA-P and ORTHROS) at a number of clinical conferences throughout the year, including AANS/CNS in February, AAOS in March, IMAST and AANS in April, ISASS in June, and other events, including NASS in October, where Camber will be hosting multiple clinical presentations.
“We now offer interbody options for anterior column reconstruction from every possible angle,” said Seth Anderson, Camber’s Chief Innovation Officer. “To support this, education and innovation continue to be our top priorities. Our patented and award-winning technology platforms will be expanding significantly this year, so hosting a broad selection of presentation and training opportunities with thought leaders is timely. This is likely to be our most productive year to date in terms of product launches and hands-on training.”