April 8, 2021
Carmafil, LLC is excited to announce the launch of a newly designed guidewire bowl that helps specialists be more efficient during endovascular procedures.
Developed by vascular/endovascular neurosurgeon Dr. Cargill Alleyne, this uniquely designed product is now available for commercial use.
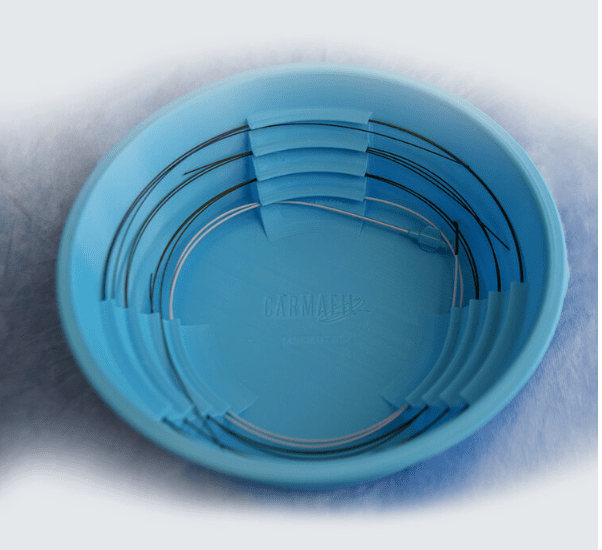
A variety of doctors (neurosurgeons, neurologists, radiologists, cardiologists, vascular surgeons, and cardiac surgeons) use catheters and wires to fix blood vessel problems in the body through a minimally invasive approach. These filaments are housed in a bowl filled with saline to keep them hydrated. The patent-pending Carmafil guidewire bowl solves the problem of having a tangle of wires and catheters at the bottom. The new bowl has a staircase design along the inside wall which segregates the wires to make it easier to retrieve them. Other key features are:
– It holds up to 4 wires or catheters
– The wires or catheters can easily be retrieved with one hand
– It reduces potential contamination during retrieval
– The light blue color facilitates visualization of the wires in dim lighting
– It was designed by a practicing neuro interventionalist and an engineer.
“As a busy clinician, I’m constantly seeking ways to increase efficiency in the operating room and the angiography suite. This modification to the standard guidewire bowl goes a long way toward that end” says co-inventor and company CEO Dr. Cargill Alleyne. He is a dually trained, vascular/endovascular neurosurgeon in private practice at University Hospital Augusta Back in Augusta, GA. Dr. Alleyne is the former Chairman and Residency Program Director of the Department of Neurosurgery, Medical College of Georgia, Augusta University. The other co-founder Mahmoud Baniasadi, Ph.D. is an Assistant Professor in the Allen E. Paulson College of Engineering and Computing, Department of Manufacturing Engineering at Georgia Southern University.
Carmafil, LLC manufactures a unique guidewire bowl which was developed from the collaboration of a dually trained open/endovascular neurosurgeon and an engineer.
The company has been nationally certified as a Minority Business Enterprise by the Georgia Minority Supplier Development Council.