January 27, 2021
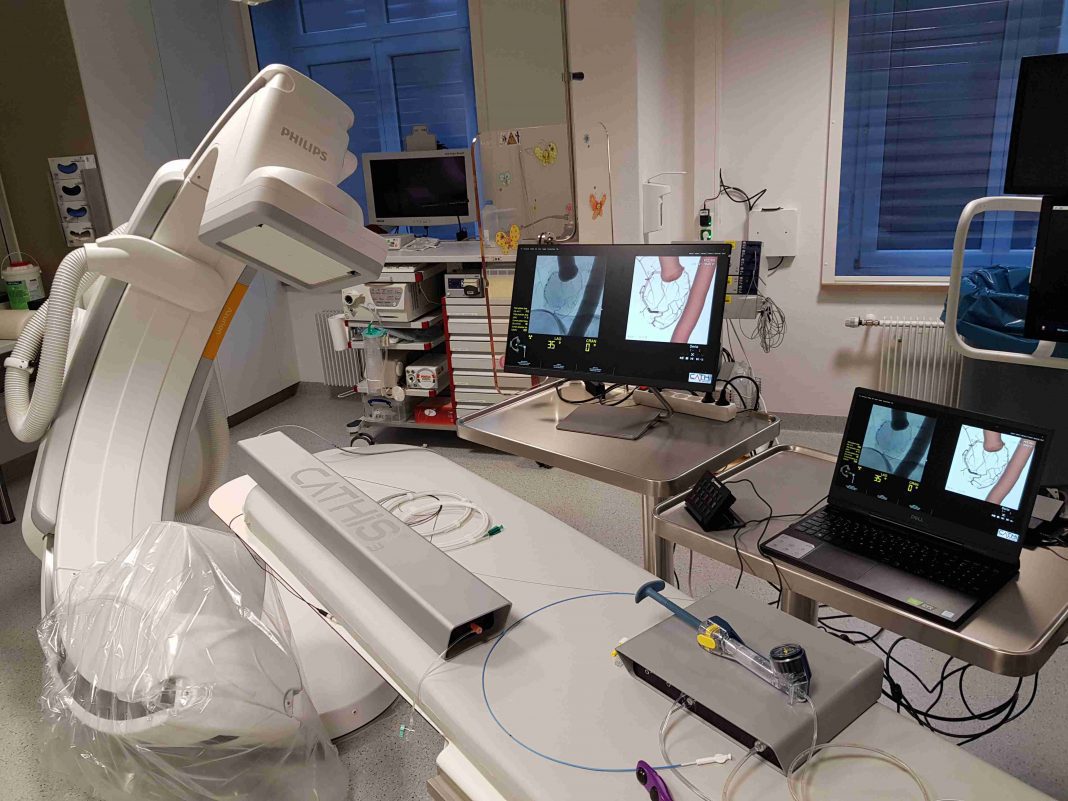
CATHIS Smart displays all types of endovascular intervention in any medical field, however complex and challenging they may be. The system is particularly appropriate for neuroradiology, cardiology, radiology, angiology and pneumology.
Being the size of a laptop, CATHIS Smart is truly transportable, but its compact dimensions do not mean that compromises have been made on either applications or features. It can be completely customised to satisfy individual customer needs, such as being set up with specific devices or instruments, along with associated special features.
CATHIS® Smart enables sales teams to give simplified yet high-definition demonstrations of complex products to their customers. They can quickly learn simple-to-follow step-by-step procedures to gain a basic understanding of vessel anatomy plus the handling of instruments or devices. CATHIS® Smart can also be used by engineers as a tool for aiding product design by facilitating visualisation and the extrapolation of ideas, as well as factoring in ease of operation and a positive user experience.
CATHIS® Smart provides a higher degree of user flexibility than competing simulators: rather than having to run through the entire procedure every time they want to reach a particular section, operators can jump directly to specific steps, which is ideal for educational purposes.
“Simulators are essential items of equipment for the cath lab, both for initial training and maintaining vital skills”, said Prof Andreas Götte, Head of Cardiology at the St Vincenz Hospital, Paderborn, Germany. “But to be effective they must resemble ‘real life’ clinical practice: we must see the coronary artery as it really is, being able to manipulate guide wires as well as catheters and balloons.”
“CATHI is a small German-based independent supplier of endovascular simulators”, added Manuela Werner, Managing Director of Marketing and Sales. “We design and manufacture all our software and hardware in-house, which means that we can provide completely customised systems to extremely high specifications. We also offer unique features not found on any other systems, such as the deployment of liquids”.
CATHIS® Smart is an innovative, portable high-quality simulator, which in combination with the appropriate software modules is applicable to a wide range of specialist applications. The CATHIS® Smart simulator is supported by a range of hardware including a control unit, notebook and foot pedal.