Clinical Research
By Harsha Rajasimha, Ph.D.
Rare diseases present a significant challenge to our society and healthcare systems. Globally, some 8,000 rare diseases have been identified, of which only 7% are treatable. More than 93 percent of rare diseases currently have no approved treatment. Although rare diseases may be rare individually, they are collectively common, with 1 in 17 people being affected by one of 8,000-plus rare diseases at some point in their lives.1
More than 30 million (nearly 1 in 10) Americans are estimated to be living with a rare disease; half of them are children. There are also shortcomings in our understanding of rare conditions. Though rare diseases are of low prevalence, and they are individually rare, collectively they affect a considerable proportion (between 6% and 8%) of the population in any country. Rare diseases include rare cancers, degenerative diseases, genetic disorders, cardiovascular diseases, and infectious tropical diseases.
Though there is no universally accepted definition of rare diseases; different countries define these diseases differently. Recently, large pharmaceutical companies across the world have begun to pay more attention to rare diseases due to the incentives offered by governments. Pharmaceutical companies and clinical research organizations are advancing rare diseases research in a number of ways from identifying, recruiting, and retaining patients to discovering signs of diseases to accelerating diagnosis, and delivering insights into complex patient populations.
Raising awareness about rare medical conditions
Every year, February 28 is celebrated as International Rare Disease Day, organized to raise awareness of the rare diseases and genetic conditions for 350 million patients worldwide. Today, advanced medical technology and the latest research capabilities allow doctors to diagnose many of the rare disease conditions prenatally or at birth, while others are diagnosed later in life.
Globally, some diseases are so rare that they are rarely diagnosed and remain undiagnosed over several years. Rare diseases affect not only the patients but also their families. Often, families with rare conditions feel isolated—they do not know where to find resources, how to get a diagnosis and treatment options. The rare disease diagnostic journey is often long with visits to numerous specialists. A large proportion of these patients gets misdiagnosed multiple times and spend years with multiple physicians for diagnosis who are not aware of their disease, screening, and treatment.
More education and awareness are needed about rare diseases among healthcare providers as well as the public. If patients and families come together to advocate for disease awareness, more money could be raised for better research. This will encourage R&D investments by biopharmaceutical companies to develop therapies for rare diseases.
Massive global health inequities in rare diseases
Health inequities stem from the systematic differences in the availability of opportunities for healthcare, leading to avoidable differences in health outcomes for different individuals. Social determinants of health including age, income level, gender, employment status, geographic location, race, and ethnicity contribute to this inequity and impact the health of an individual.
Health inequity is due to some structural and human-made systems which are beneficial to certain groups of people while underserving others. This is due to the unequal distribution of power and resources. These structures involve ethnicity, which gives more power and resources to one ethnic group over another; gender discrimination, which favors a certain gender; classicism, which gives an advantage to those with wealth and social status; ableism, which values able persons over mentally or physically disabled, and many others.
The resulting health inequities affect various levels, including internal, interpersonal, and institutional. These influence social, economic, and environmental differences which directly impact patient’s health, and lead to health inequities, which leads to deteriorating outcomes.
As a result of these health inequities, individuals with rare diseases experience
- Increased difficulty in access to treatment
- Increased stress and anxiety
- Decreased productivity and employment.
The healthcare system loses billions of dollars every year further increasing the overall cost of healthcare for all.
What is being done to develop treatment for rare diseases?
In 2021, the National Organization of Rare Disorders (NORD), Global Genes, and other organizations collaborated with Rare Disease Diversity Coalition to identify causes of health inequities. The researchers have made progress to identify, treat, and protect people from a variety of these diseases. However, there is much more to do as most rare diseases across the world still have no treatments.
Efforts to improve and bring to market treatments for rare diseases are coordinated by FDA. America’s biopharmaceutical companies are leveraging the latest technologies, and growing understanding of the genetic cause of many rare diseases to develop the best and groundbreaking therapies. In 2020, the FDA and CDER (Council for Drug Evaluation and Research) approved approximately 31 drugs to treat rare diseases.
In addition to drugs and biologics, the development of medical devices is also being done for rare diseases. Since 1990, the FDA’s Center for Devices and Radiological Health (CDRH)2 has approved 78 medical devices for rare diseases diagnosis and treatment.
One such device, approved in 2020, reduces coronary artery atheroma in adult patients with Homozygous Familial Hypercholesterolemia (HOFH), who are either intolerant or irresponsive to the maximal therapy of HOFH. This is a rare genetic disorder in which the patients develop significantly elevated bad or LDL cholesterol and have increased risk of heart diseases.
CDRH is making efforts continuously with stakeholders from across the medical devices world to meet the demands for medical technology. Apart from this, clinical research organizations are making efforts by communicating with patients. The FDA has collaborated with scientists, policymakers, patients, medical devices developers, and patient advocacy groups to address the challenges associated with rare diseases treatment. The FDA patient listening sessions were created in recent years to provide an important forum for patients from around the world to have their voices heard. Especially, it is important for therapy developers and regulators to understand what endpoints matter to patients and which do not matter as much. The FDA is increasingly realizing that assessing the safety and efficacy of orphan products calls for the adoption of different standards when compared to other products.
How can people with rare diseases help to advance progress for their conditions?
Patients with rare diseases should become advocates or even warriors for their own cause. They can move the needle on all fronts to accelerate therapy development process. Direct engagement of patients in the research plan can result in a positive and productive relationship for all members of the team. Research and clinical trials are showing interest in involving patients actively to ensure the best outcomes. If patients with rare diseases are involved right at the beginning of the clinical development process, it will significantly improve the study design and delivery.
Working with patients or advocacy groups can be helpful in guiding patients through the treatment process, social support, understanding clinical trials process, and financial assistance. Patients with rare diseases play an important role in helping the healthcare provider make an accurate diagnosis and for therapy discovery and development. These roles include:
- Becoming knowledgeable about the illness and treatment
- Joining a trusted patient advocacy organization
- Enrolling into a patient registry or natural history study
- Knowing about all medications along with dosage details
- Asking questions and writing information for further use
- Knowing the rights as a patient.
Global collaboration for accelerating clinical studies
Clinical trials are crucial to developing the treatments discovered in research labs and helping them to reach the patients. The regulatory authorities like FDA require these studies to demonstrate a therapy’s effectiveness and safety with sufficient statistical significance prior to approval.
Several organizations in the US such as Greater Gift and Rare Disease Diversity Coalition are focused on addressing the massive health inequities & disparities within the country. There is a growing recognition that clinical trials have to include patient populations that are globally representative of the people targeted by a drug or vaccine. Historically, there has been a significant dearth of global diversity among clinical trial participants. As indicated in the map of where clinical trials (all diseases combined) are concentrated in the western world in the map below taken from clinicaltrials.gov as of April 14, 2022.
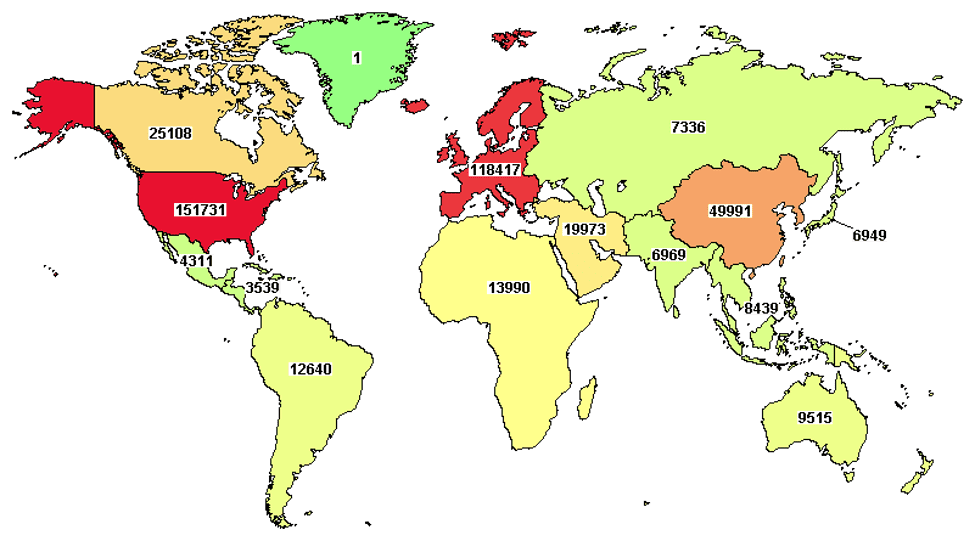
The map highlights the huge unmet need to foster cross-border collaborations and engaging the populous regions of the world such as the Indian subcontinent. This will enable better engagement, enrollment of patients in that region that will help accelerate clinical trials as timely recruitment of patients is the single biggest barrier to faster therapy development. This is where Indo US Organization for Rare Diseases aims to make an impact through strategic collaborations with patient advocacy groups, industry partners, researchers, policy makers, and other stakeholders in India and the US.
How patient advocacy is empowering patients?
Patient’s wellbeing, proper care, and treatment are central to the healthcare sector, no matter what the level of disease or illness is. Patient advocacy is an integral part of the healthcare, which ensures a patient’s rights and concerns. Disease-specific patient advocacy organizations are well supported by umbrella organizations such as the National Organization for Rare Diseases (NORD), Global Genes, Every Life foundation for rare disorders, and Indo US Organization for Rare Diseases, in educating patients with rare diseases, and advocate for them to their local, national, and international communities including legislators. The role of any government group or agency as a patient advocate enables it to:
- Connect with other patients and stakeholders
- Share stories to raise awareness
- Educate the patient
- Develop good relations with key decision-makers
- Participate in regional and local events to connect
- Explain medical terms to the patient
- Mediate between patients and doctors
- Review care and treatment plans
- Connect patients to critical resources, stakeholders, and assets.
Conclusion
Raising awareness about rare diseases means making people conscious of them. The goal of risk awareness is to make people understand the importance of their condition. Early diagnosis of treatment, regular monitoring, and better education via patient advocacy are crucial to deal with the challenges associated with rare disease management.
People with rare diseases deserve to get the same level of diagnosis, care, and treatment as those with common conditions. Rare disease expertise is distributed globally and hence requires global collaboration across international borders to advance and accelerate progress for all patients.
References
- https://www.fda.gov/news-events/fda-voices/fda-working-bridge-gaps-and-meet-needs-rare-disease-product-development
- https://www.fda.gov/about-fda/fda-organization/center-devices-and-radiological-health
Editor’s Note:
Harsha Rajasimha, Ph.D., Founder, Chairman of the Board and CEO. Dr. Rajasimha is the founder and chairman of the humanitarian non-profit Indo US Organization for Rare Diseases. Earlier, he served as cofounder and co-chair of the Organization for Rare Diseases in India from 2013 to 2019. He has been academically affiliated as a faculty member in the School of Systems Biology at George Mason University in Fairfax, VA since 2012. He is also the founder and CEO of the decentralized clinical trials software company, Jeeva Informatics Solutions, based in Virginia. He is a precision medicine data scientist-turned social entrepreneur on a mission to accelerate human-centric clinical research through technology innovation and global advocacy.
Dr. Rajasimha has more than a decade of experience working on various interdisciplinary projects involving genomics and big data as a consultant for clients including the National Cancer Institute, National Eye Institute, Georgetown University, and Genome International Corporation. His research has focused on the genomics and systems biology of diseases including cancer, infectious diseases, neuro-muscular diseases, and retinal degenerative diseases. He completed his M.S. in Computer Science and Ph.D. in Genetics, Bioinformatics and Computational Biology at Virginia Tech, where he developed and applied reusable simulation models of mitochondrial DNA heteroplasmy dynamics to study various diseases.
