Covalon Technologies Ltd. (the “Company” or “Covalon”) (TSXV: COV; OTCQX: CVALF), an advanced medical technologies company, today announced its participation in the Infusion Nurses Society Annual Meeting and Exhibition, to be held in Kansas City, Missouri from Saturday, May 18 to Monday, May 20, 2024. Visitors to Covalon’s booth (#420) will have the opportunity to explore the Company’s innovative vascular access and infection control solutions first-hand and learn why many top hospitals are choosing Covalon solutions for their patients.
“One of our primary goals is to give patients a feeling of freedom during infusion treatments – freedom from the worry of painful dressing removals and skin injuries,” said Brent Ashton, Chief Executive Officer, Covalon Technologies. “Our dressings are unique due to their exceptional coverage, composition, and chemistry. Our state-of-the-art silicone-based vascular access dressings merge safety and efficacy with empathy, moving us closer to providing patient care that is as compassionate as it is effective.”
Not all vascular access dressings are made the same, and Covalon Technologies is excited to showcase more compassionate solutions at the Infusion Nurses Society Annual Meeting and Exhibition. The event brings together hundreds of infusion therapy professionals from all over the globe to learn about the latest improvements to patient care and see the newest products on the infusion market. It offers a unique opportunity for healthcare providers to engage with leading-technologies and enhance their practice with the latest innovations.
Covalon’s patented vascular access products include:
VALGuard® – a transparent, environmental barrier designed to protect catheter hubs and line connections from external contaminants and gross contamination, including body fluids and other secretions. It incorporates a quick-release pull strip for fast access to infusion hubs and for easy removal.
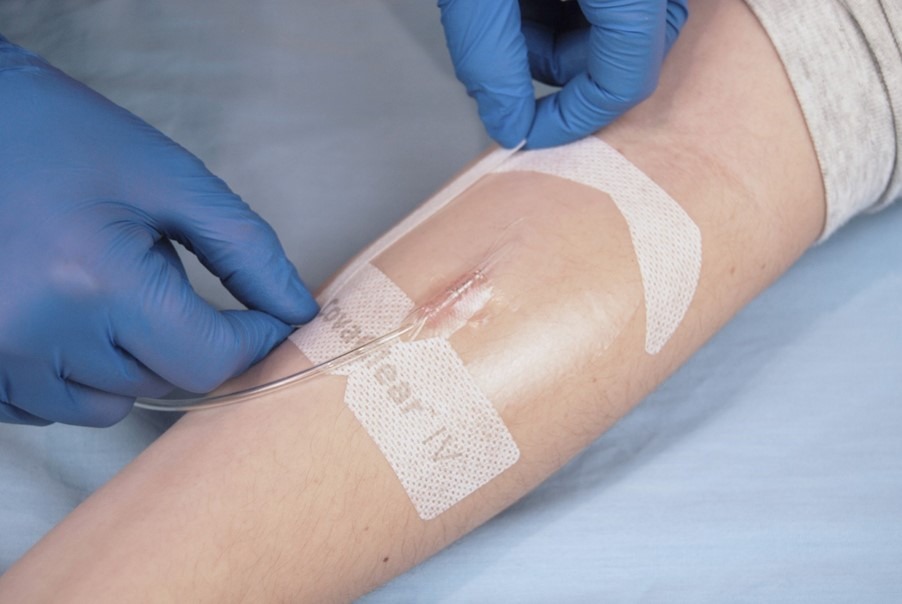
IV Clear® – the world’s only dual-antimicrobial vascular access dressing that offers complete transparency at and around the insertion site for easy daily assessment and utilizes gentle silicone adhesive technology to help protect skin from injuries.
CovaClear® IV – utilizes soft silicone adhesive technology to help protect patients from skin injuries, but does not incorporate antimicrobials, for use with patients who either do not require or cannot tolerate antimicrobials.