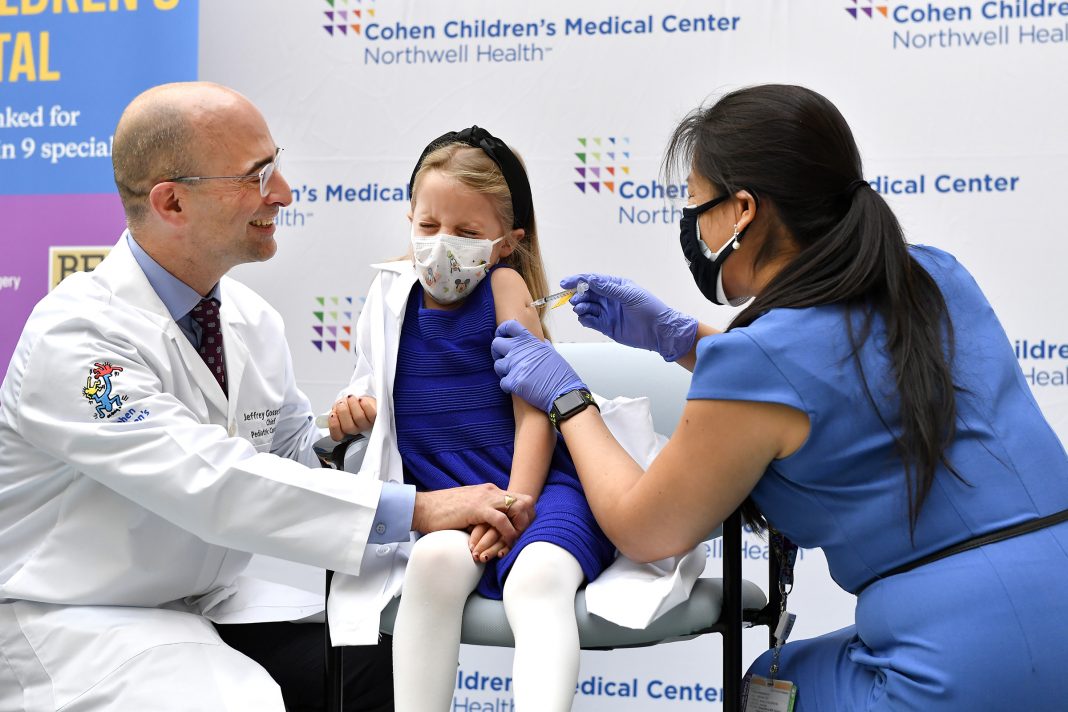
Children ages 5-11 and their parents are one step closer to ending the pandemic after Cohen Children’s Medical Center today began offering Pfizer-BioNTech’s kid-size coronavirus disease 2019 (COVID-19) vaccine. The Center for Disease Control and Prevention signed off on the latest age group to be vaccinated – representing 28 million Americans – and clearing the way for the families of seven youngsters to be the first at Northwell Health.
The patients and their parents, many of whom are physicians at the hospital, were greeted by Charles Schleien, MD, senior vice president and chair of pediatrics at Northwell Health and Cohen Children’s. Noting that this vaccination was a big step toward protecting those who are eligible, Dr. Schleien said, “In New York, six in 10 kids who are 12-and-over are already vaccinated. We need to get that number higher – and starting today, we need to chip away at vaccinating the newest group of eligible children. The vaccine is safe. It works – and it will be the way we successfully lower COVID stats across the region, nation and world.”
Sophia Jan, MD, chief of general pediatrics at Cohen Children’s, was on hand to vaccinate the youngsters and offer her support to their parents. “Being vaccinated will make it so much easier for our youngsters to get back to their normal lives,” said Dr. Jan. “Not only will they be able to get back to school and enjoy the holidays, but the important things – like hugging their parents and visiting their grandparents – we can start thinking about these things again.”
When interviewed as a group by a roomful of reporters, the newly vaccinated children all agreed that a moment of discomfort was well worth the relief of staying virus free. “Maybe one day we can go to school without masks,” they said.
In an effort to provide vaccinations to this newest eligible group, Northwell Health has established vaccination pods in Manhattan, Queens, Long Island and Westchester County. Locations and hours are:
- Nassau County: (1 Marcus Ave, New Hyde Park, NY) Nov. 7 & 14, 9 a.m.-5 p.m.
- Suffolk County: (5181 Sunrise Highway, Bohemia, NY) Nov. 6 & 13, 9 a.m.-5 p.m.
- Suffolk County: (284 Pulaski Road, Greenlawn, NY) Nov. 11, 8 a.m.-8 p.m.; Nov. 18, Noon-8 p.m.
- Suffolk County: (4 West 2nd Street, Riverhead, NY) Nov. 7 & 14, 9 a.m.-5 p.m.
- Manhattan: (200 West 13th Street, New York, NY) Nov. 10, 3-7 p.m.; Nov. 13, 10 a.m.-3 p.m.
- Queens: (95-25 Queens Blvd., Rego Park, NY) Nov. 6 & 13, 9 a.m.-9 p.m.
- Westchester County: (480 North Bedford Road, Chappaqua, NY) Nov. 7 & 14, 9 a.m.-5 p.m.
To schedule a vaccine appointment, call (844) 919-VACC for the most up-to-date availability or go to: https://www.northwell.edu/coronavirus-covid-19/vaccine/locations.