February 1
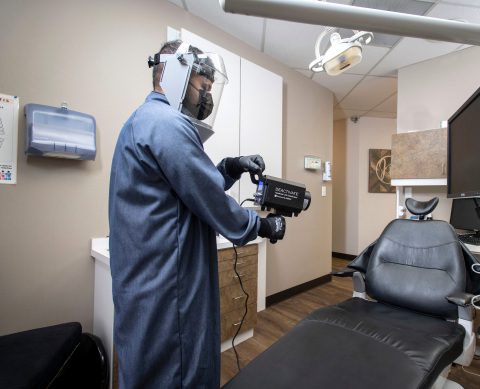
Deactivate is a high-powered, handheld LED device designed to quickly disinfect surfaces in confined spaces.
Xenex is known for its LightStrike™ Germ-Zapping Robots™, which have been deployed by hundreds of healthcare facilities worldwide for no-touch room disinfection. As a result of the COVID-19 pandemic, LightStrike robots are now used in airports, schools, hotels, sports arenas, police stations and correctional facilities, convention centers, and more to quickly disinfect large rooms and areas.
Recognizing the need for targeted disinfection in small spaces and compact areas, Xenex has launched Deactivate, which utilizes high-powered LEDs to create ultraviolet (UV) light proven to deactivate pathogens, including severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes COVID-19. Deactivate does not require warm-up or cool-down time, and does not leave behind any chemical residue. Post-disinfection, the area is immediately available for use.
“Our mission is to stop the pain and suffering caused by infections by destroying the pathogens that cause them. There are many businesses that need targeted, rapid disinfection, especially compact areas like dental exam rooms, ambulances, office cubicles, and cockpits. We wanted to offer an effective technology for disinfecting small spaces that are hard to clean and that’s what Deactivate provides,” said Irene Hahn, senior vice president of sales and marketing for Xenex.
Evidence-based. Testing performed at the Texas Biomedical Research Institute demonstrated that the Deactivate device achieves a 99% level of disinfection against SARS-CoV-2 in 30 seconds at 1 meter, 99% against vegetative bacteria (methicillin-resistant Staphylococcus aureus – also known as MRSA, Escherichia coli – also known as E.coli) in 1 minute at 1 meter, and 99% against bacterial spores in 2 minutes at 1 meter.