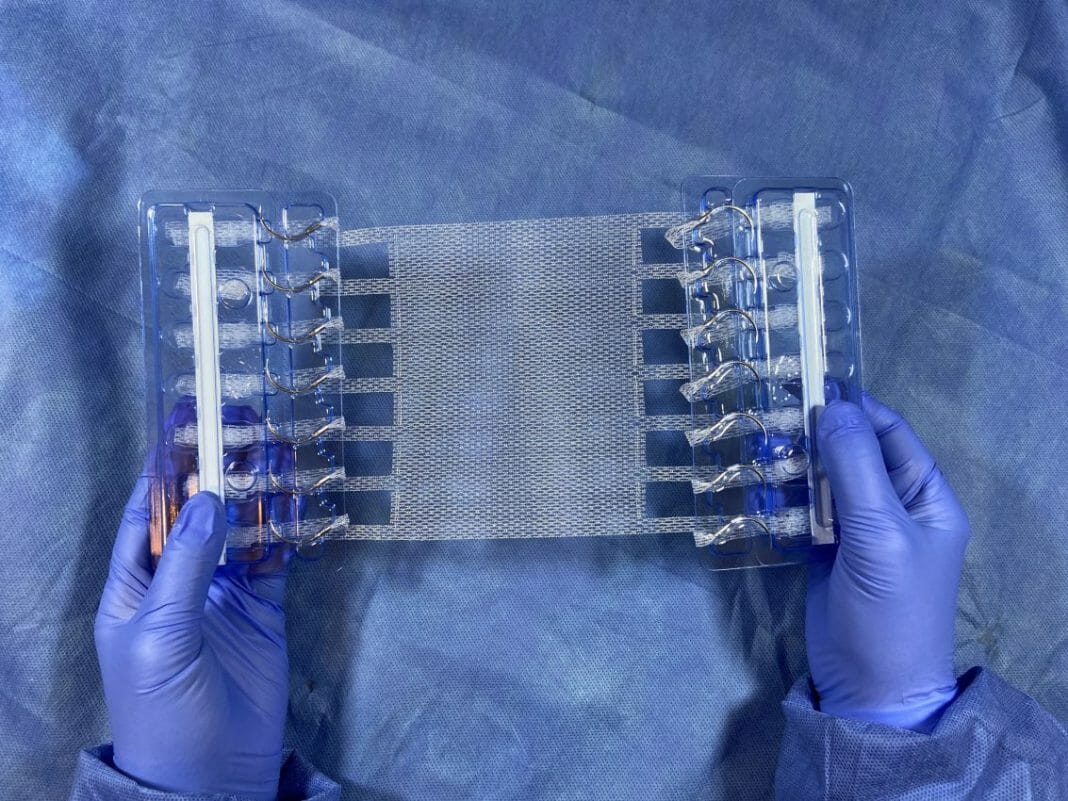
Deep Blue Medical Advances announced today it has received an additional 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its T-Line® Hernia Mesh for the sublay technique in open hernia surgery. This significantly expands the patient population that can be treated with T-Line and experience the unique benefits of its novel design and application.
The sublay procedure is the most widely performed open surgery ventral hernia repair method for large incisional hernias. With this clearance, the T-Line Hernia Mesh is indicated for the reinforcement of soft tissue where weakness exists for the repair of ventral hernias performed via an open onlay or sublay approach in adults.
Deep Blue’s T-Line mesh design includes novel mesh extensions that provide superior anchor strength designed to prevent mesh fixation failure. T-Line’s design offers the surgeon the ability to provide optimal mesh tension adjustment and many other potential surgical and clinical advantages compared to conventional mesh application.
“Many hernia surgeons prefer the sublay technique for hernia repair and have been eager to use T-Line for this, so we are thrilled to receive this clearance to enable broader clinical use,” said Dr. Howard Levinson, Deep Blue’s founder and Chief Medical Officer.
“There are 4–5 million abdominal incisions (laparotomies) performed annually in the United States with hernias resulting after approximately 25% of these procedures1. Further, long-term ventral hernia repair failure rates are up to 32% using conventional mesh and 63% with suture repair only, creating a multibillion-dollar clinical cost to the US healthcare system2. Consequently, there are over 400,000 incisional hernia repairs performed each year in the United States making it one of the five most common procedures performed by general surgeons1,” said CEO Bill Perry. “This expanded clinical indication allows our growing number of clinical sites to address this important problem in significantly more patients with T-Line Hernia Mesh.”
- Harris HW Innovations for Incisional Hernia Prevention, J. of Abdom. Wall Surg. (2022) 1:10945
- Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. (2004) Oct;240(4):578-83