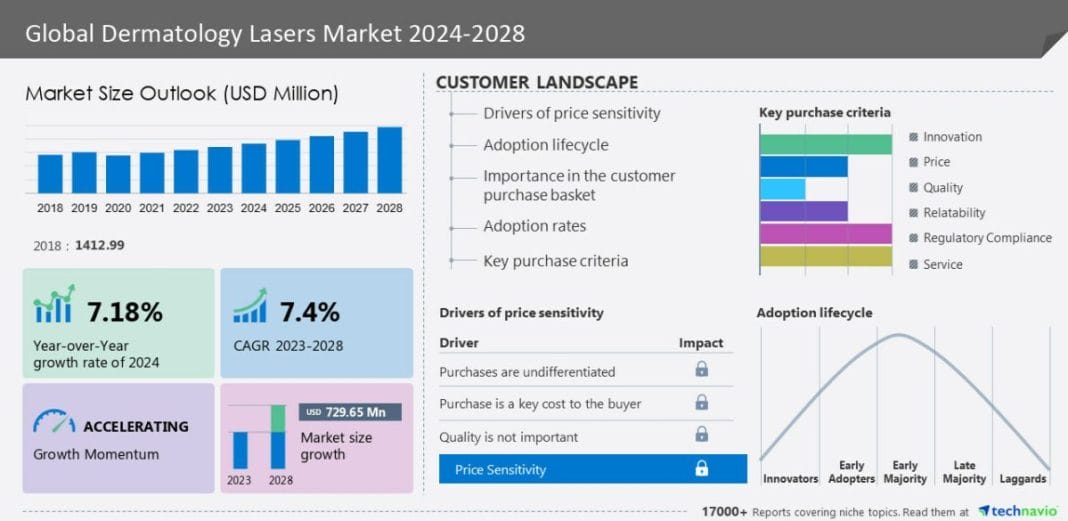
The dermatology lasers market size is expected to grow by USD 729.65 million from 2023 to 2028. In addition, the momentum of the market will progress at a CAGR of 7.4% during the forecast period, according to Technavio Research. The market is categorized by End-user (Dermatology clinics, Hospitals, and Others), Product (Solid-state dermatology lasers, Gas dermatology lasers, and Pulsed dye dermatology lasers), and Geography (North America, Europe, Asia, and Rest of World (ROW)).
North America will contribute 39% to the growth of the global market during the forecast period. The region is the largest revenue contributor to the global dermatology lasers market. This can be attributed to the increasing awareness and adoption of non-invasive medical skin treatments in the region. The low cost of surgeries in the country makes Mexico a leading country in terms of aesthetic surgeries, with more than 900,00 procedures performed annually. This report offers an up-to-date analysis of the current market scenario, the latest trends and drivers, and the overall market environment. Read a PDF Sample Report
Company Profile:
Abbott Laboratories, Asclepion Laser Technologies GmbH, Bausch Health Companies Inc., biolitec AG, Boston Scientific Corp., Candela Corp., Canfield Scientific Inc., Carl Zeiss AG, Cutera Inc., Cynosure LLC, Fotona d.o.o, IPG Photonics Corp., KRUPA MEDI SCAN, Miracle Laser and Skin Care, Quanta System S.p.A., Sisram Medical Ltd., Strata Skin Sciences Inc., Gavia Asesores Ayala SLU, Coherent Inc., Koninklijke Philips N.V.
Abbott Laboratories – The company offers dermatology lasers such as Paul Getz, MD, CellFX, and others.
To gain access to more vendor profiles available with Technavio, buy the report!
Dermatology Lasers Market: Segmentation Analysis
The dermatology clinics segment will be significant during the forecast period. Laser hair removal, skin resurfacing, scar revision, and vascular lesion removal are carried out in these clinics. Such treatments require specialized equipment and expertise, which dedicated dermatology clinics can provide. Learn about the contribution of each segment summarized in concise infographics and thorough descriptions. View a PDF Sample Report
“Besides analyzing the current market scenario, our report examines historic data from 2017 to 2021”- Technavio
Dermatology Lasers Market: Driver & Trend:
Drivers
- Increasing prevalence of skin conditions
- Advancements in Laser technologies
- Rising demand for aesthetic procedures
The prevalence of a variety of skin conditions around the world leads the market growth. Acne, Psoriasis, Eczema, and Rosacea are some of the conditions becoming increasingly prevalent, prompting the need for more sophisticated treatments such as dermatology lasers.
Increasing demand for minimally invasive cosmetic procedures is an emerging market trend. Identify key trends, drivers, and challenges in the market. Download a sample report to gain access to this information.
Related Reports:
The Dermatology Market size is estimated to grow by USD 12,761.97 million at a CAGR of 10.82% between 2022 and 2027.
The hair transplant market share is expected to increase by USD 9.89 billion from 2021 to 2026, and the market’s growth momentum will accelerate at a CAGR of 9.97%.
What are the key data covered in this dermatology lasers market report?
- CAGR of the market during the forecast period
- Detailed information on factors that will drive the growth of the dermatology lasers market between 2023 and 2028.
- Precise estimation of the dermatology lasers market size and its contribution to the market in focus on the parent market
- Accurate predictions about upcoming trends and changes in consumer behavior
- Growth of the dermatology lasers market across North America, Europe, Asia, and ROW
- A thorough analysis of the market’s competitive landscape and detailed information about vendors
- Comprehensive analysis of factors that will challenge the growth of dermatology lasers market vendors.