East End Medical, a private medical device company committed to improving catheter-based cardiac procedures, today announced the first commercial uses of its SafeCross™ Transseptal Radiofrequency (RF) Puncture and Steerable Balloon Introducer System.
East End Medical notes the All-in-One system is designed to provide a predictable and safe solution for performing electrophysiology and structural heart interventions requiring left atrial access.
The first commercial devices were implanted during Left Atrial Appendage Closure (LAAC) and Transcatheter Mitral Valve Edge-to-Edge Repair (TEER) procedures at New York Presbyterian Hospital -Columbia, performed by Dr. Martin Leon, Professor of Medicine at Columbia University Medical Center (CUMC), Dr. Torsten Vahl, Attending Physician at the Structural Heart & Valve Center, and Dr. Rebecca Hahn, Director of Interventional Echocardiography at the Columbia Structural Heart & Valve Center. Atrial Fibrillation (AF) and Ventricular Tachycardia (VT) ablation procedures using the SafeCross System were also performed at The Mount Sinai Hospital, New York by Dr Vivek Reddy, Director of Cardiac Arrhythmia Services, and Dr Srinivas Dukkipati, Director of the Electrophysiology Laboratory.
“Accessing the left atrium in a safe and precise manner is critical for optimal patient outcomes in left atrial procedures. Depending on the anatomy, achieving safe and precise transseptal access frequently leads to decreased overall left atrial procedure times,” stated Dr. Torsten Vahl. “In using SafeCross, I particularly liked how the balloon-tipped sheath allowed me to safely glide across the septum in small and precise adjustments to enable me to position on the exact pre-determined location of the transseptal puncture.”
“The highly visible steerable balloon introducer enables precise puncture site targeting and provides the support needed for challenging anatomies,” stated Dr Vivek Reddy. “Additionally, the ability to use it as a steerable guiding sheath makes it a nice potentially all-in-one tool for my workflow. I look forward to incorporating SafeCross into my left-atrial procedures.”
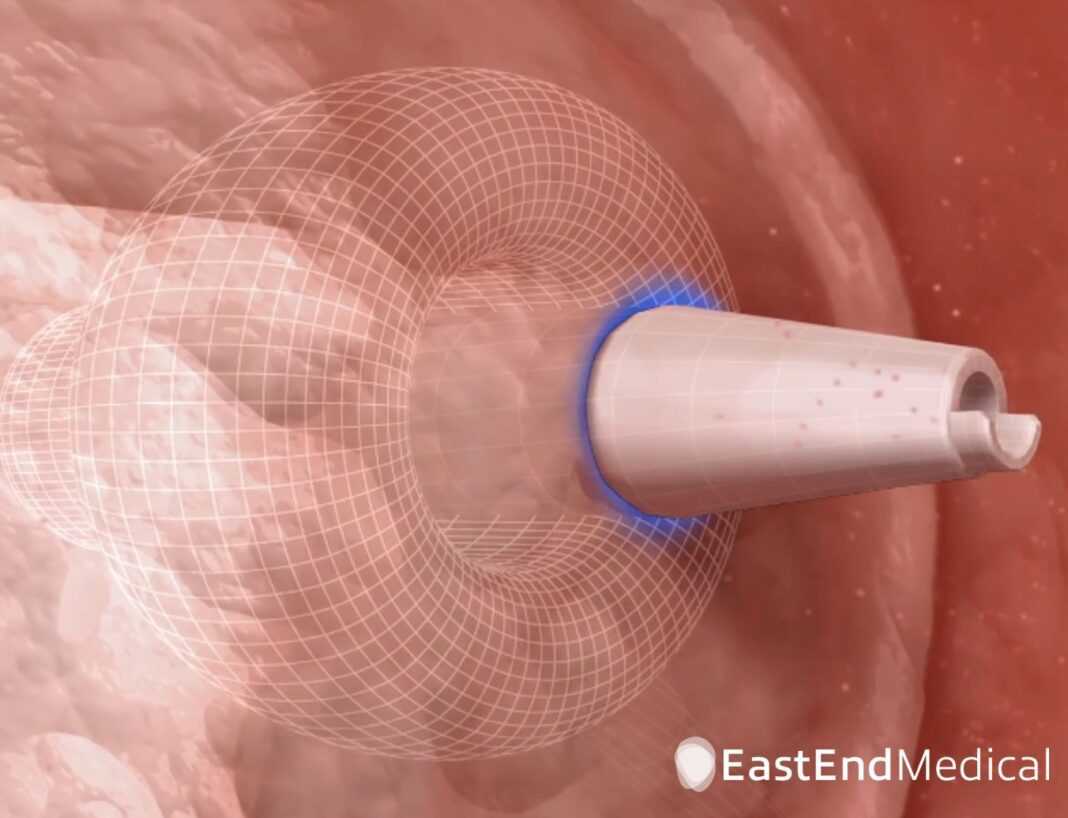
The All-in-One SafeCross System consists of a bi-directional steerable introducer sheath and a radiofrequency puncture dilator, making it a needle-less system. Uniquely designed with an atraumatic contrast-filled positioning balloon on its distal end, the introducer sheath is ultra-visible under echocardiography and x-ray to facilitate precise puncture site selection, while its bi-directional steerability allows for safe maneuvering and perpendicular placement on the septum. The proprietary RF puncture dilator delivers electrical energy quickly and predictably to puncture the atrial septum.
“As a structural heart interventional echocardiographer who helps guide these procedures, the steerable balloon sheath and needle-less RF system provides for a very visible, precise, and safe transseptal experience,” says Dr Rebecca Hahn. “The precision and efficiency with which the interventionalist can now perform the transseptal puncture is remarkable and will likely shorten procedure times and improve procedural success.”
“With the increasing number of left atrial procedures, it is imperative to have a left atrial access device that helps physicians safely and predictably cross the septum,” commented Dr. Martin Leon, “The SafeCross system represents a novel tool for successful left atrial procedures including left atrial appendage closure (LAAC), transcatheter mitral valve repair (TEER), and atrial fibrillation (AF) ablation procedures among others. I look forward to collecting clinical evidence on the performance of the SafeCross system and to focusing on training initiatives to improve the safety and efficacy of left atrial procedures.”
“We are excited to have successfully completed the first commercial cases of the SafeCross system, a significant milestone for our company,” said Anthony Medigo, Chief Commercial Officer of East End Medical. “The SafeCross system brings several benefits to physicians performing left atrial procedures and we look forward to bringing this important technology to more hospitals in the U.S. market later this year.”