March 25, 2020
Enrollment in Tria LifePolymer study includes fifteen patients’ reports FOLDAX® at four participating sites.
“The study is progressing well and we will be reporting on the early clinical experience later this year,” stated Amit Patel, MD, MS, the principal investigator of the study.
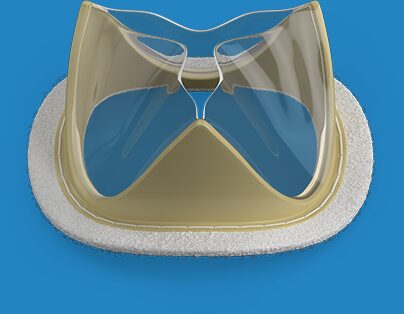
The aortic EFS is the first in a series of clinical studies Foldax will be conducting on its Tria LifePolymer heart valve line. LifePolymer is a patented polymer formulated specifically for heart valve application with the goal of enhancing durability, hemodynamic performance and providing exceptional value over current heart valves. The next studies will focus on surgical mitral valve and transcatheter aortic valve applications.
“This accomplishment would not be possible without the significant contributions of our clinical investigators and their teams at Ohio Health in Columbus, The Christ Hospital in Cincinnati, St. Vincent’s in Indianapolis and Beaumont Hospital in Detroit. We are looking forward to initiating our surgical mitral and TAVR clinical studies to continue our goal of transforming heart valve therapy,” stated Ken Charhut, Foldax Executive Chairman.