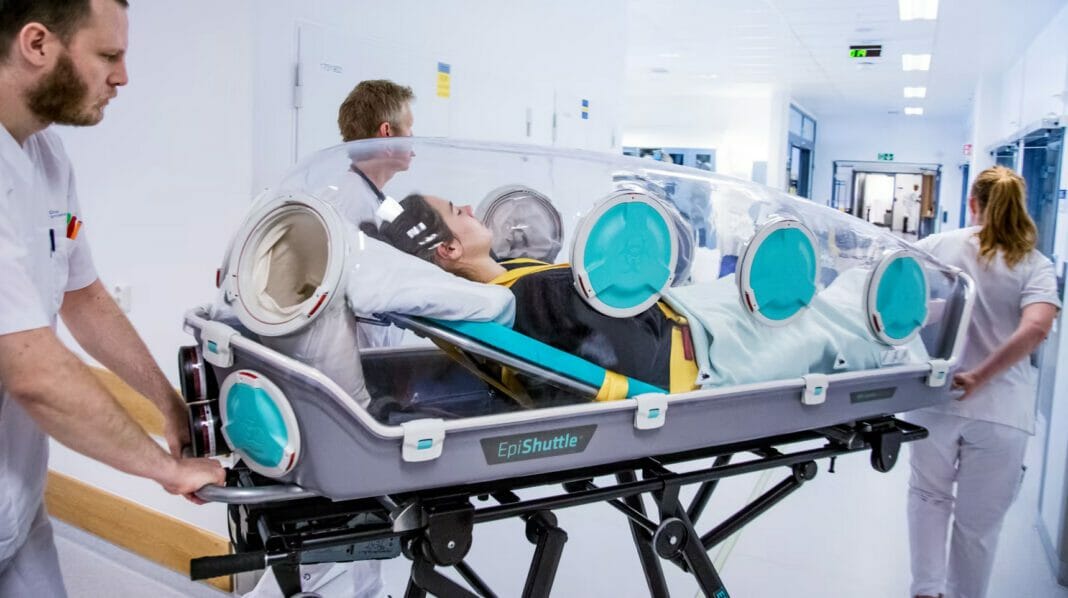
EpiGuard reports all five University Hospitals in Finland come together to implement the new isolation and transport system. With EpiShuttles at all hospitals, Finland stands better prepared to face new pandemics or chemical and nuclear threats.
EpiGuard notes as one of the actions to increase Finland’s preparedness, the five university hospital districts jointly procured the so-called EpiShuttles, a Norwegian invention that isolates a contagious patient while being treated.
We are implementing a highly efficient tool to transport patients with highly contagious diseases, or chemical, biological or other contamination necessitating isolation. The single patient isolation and transport unit are designed to provide maximum public safety while allowing critical care and treatment to be performed on the contaminated patient inside. The unit can be used for patient transport through air, land, and sea in collaboration with the Finnish Border Guard and Defence Force units when needed, keeping society safe, Tom Silfvast, Chief Medical Officer at the Preparedness Unit at Helsinki University Hospital, said.
The procurement of the nine EpiShuttles is financed by the Government and steered by the Ministry of Social Affairs and Health. The new equipment will be used in all the areas that the five University Hospitals cover notes EpiGuard.
Ready for the next pandemic
Back in 2018, the WHO made a list of 10 diseases with the potential to cause a public health emergency. On that list was Disease X, a pathogen currently unknown. As it turns out, Disease X was the coronavirus. However, the threat of yet another new pathogen emerging, has never been greater.
With this new technology, we stand better prepared for a new pandemic. Contagious patient logistics are complicated. Finland has a lot of remote areas, and transporting contagious patients fast over long distances, into hospitals for treatment is a severe challenge. Time-consuming disinfection procedures of helicopters, aircraft, and ambulances after each transport threaten capacity. Not knowing what comes next, the EpiShuttle is our best option. We now stand ready to deploy if needed, Sanna Hoppu, Associate Professor and Chief Physician at Tampere University Hospital, said.
Safe transport of contagious patients is essential when handling the epidemic. The Johns Hopkins Center for Health Security in partnership with the World Economic Forum and the Bill and Melinda Gates Foundation recommends such medical countermeasures as the EpiShuttle represents at a “Disease X dummy run”, the so-called Event 201.
Patient transport to where ICU capacity is available has proven to be a crucial piece of the puzzle when handling a pandemic. Only when safe transport is in place can we utilize the full capacity of the entire health care system and ensure treatment for everyone, Ellen Cathrine Andersen, CEO at EpiGuard said.
The WHO R&D Blueprint Special Advisory Group said, “History tells us that it is likely the next big outbreak will be something we have not seen before.” This still goes, and a Disease Y can surface at any time.
The UN Humanitarian Response Depot (UNHRD) holds six EpiShuttles, for the safe transport of contagious patients. Congo was the first place they dispatched, during the Ebola outbreak. UNHRD offers storage at no cost at six strategically located locations near major ports and airports.
EpiGuard notes their EpiShuttles are used by WHO as well as first responders in Germany, Belgium, Denmark, Peru, Ireland, Greece, Norway, and the UK. Also, national air forces like the British, Australian, Canadian, Danish, Norwegian and others are equipped with EpiShuttles. In the private sector, EpiShuttles are a common sight amongst companies like FAI air ambulance, Air Alliance Medflight, DRF Luftrettung, Keewatin Air, Luxembourg Air Rescue, Loganair and others.