October 30, 2020
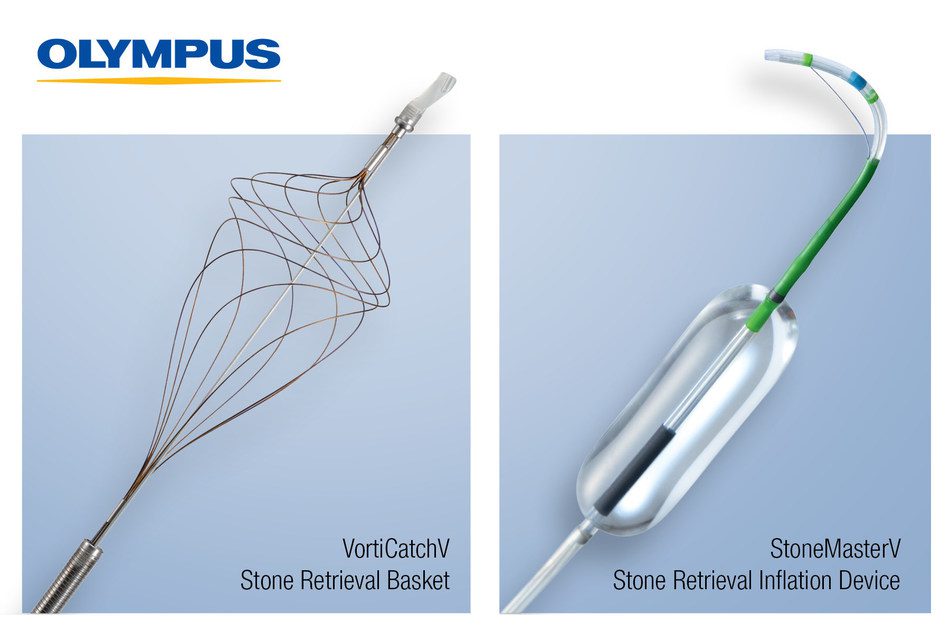
ERCP stone management devices StoneMasterV and VorticCatchV have been launched by Olympus.
ERCP stone management devices increase efficiency in bile duct stone management and retrieval for endoscopic retrograde cholangiopancreatography (ERCP).
“At the forefront of our product design and innovation is the desire to expand the capabilities of our physician customers. Both the StoneMasterV and VorticCatchV provide physicians greater opportunity to achieve better patient outcomes and increased procedural efficiency for difficult-to-reach stones,” said Kevin Mancini, Vice President for Endoscopy at Olympus America Inc. “We are proud to add StoneMasterV and VorticCatchV to our comprehensive EndoTherapy portfolio.”
ERCP is a common endoscopic procedure used to study the ducts that drain the liver and pancreas in order to identify blockages in the biliary or pancreatic ducts caused by stones, tumors or scarring.1 Therapeutic ERCP treatments include sphincterotomy, stone removal, stent placement, balloon dilation, and tissue sampling. Approximately 650,000 ERCP procedures are performed in the U.S. annually.
The StoneMasterV provides a two-in-one solution for optimized performance in endoscopic sphincterotomy and endoscopic papillary balloon dilation, prior to retrieving large stones from the bile duct. Saving time by eliminating the need for device exchange, endoscopic sphincterotomy with endoscopic papillary dilation has demonstrated a higher rate of success and fewer complications when removing stones equal to or greater than 12mm.2 This single device combines an electrosurgical knife, known as a sphincterotome, and a multi-sizing dilation balloon in one endoscopic device to help master difficult stone management.
Additional benefits of the StoneMasterV include:
- Improved Visualization: The radiopaque and endoscopic center markers provide excellent visualization and controlled positioning within the papilla.
- Enhanced Safety: The CleverCut coating at the proximal end of the cutting wire minimizes damage to the surrounding tissue, providing enhanced safety when cutting.
- Procedural Efficiency: The short wire dilation balloon with the c-channel split provides the physician control of the guidewire.
VorticCatchV is the newest addition to Olympus’ comprehensive stone retrieval basket portfolio. The single-use nitinol retrieval basket is designed for use in ERCP and is ideal for stone extraction cases in which a balloon is unable to achieve extraction, due to the stone being located in a bile duct pocket, positioned in a narrow or intrahepatic duct, or when heavy sludge removal is required after lithotripsy.
Coming in an eight-wire configuration, VorticCatchV features a spiral design that grows tighter at the distal end enabling the capture of a wide range of stones.
Benefits of VorticCatchV design include:
- Greater Flexibility: The nitinol wire material allows the basket to flex into bile duct pockets, resulting in improved stone retrieval performance and accessibility.
- Shape Memory: Nitinol is a memory alloy, which is more kink resistant than stainless steel options, allowing the basket to hold its shape more effectively.
- Maintains Full Diameter: The soft nature of nitinol wires enables the basket to fully open in narrow ducts – this ability to maintain a fully open diameter provides greater opportunity to capture difficult-to-reach stones from bile duct pockets.
References
1 “Endoscopic Retrograde Cholangiopancreatography (ERCP),” American Gastroenterological Association, August 10, 2020, https://gastro.org/practice-guidance/gi-patient-center/topic/endoscopic-retrograde-cholangiopancreatography-ercp/.
2 Kim JH, Yang MJ, Hwang JC, Yoo BM. Endoscopic papillary large balloon dilation for the removal of bile duct stones. World J Gasteroenterol. 2013;19(46):8580-8594. doi.10.3748/wjg.v19.i46.8580. 2. Rouquette O, Bommelaer G, Abergel A, Poincloux L. Large balloon dilation post endoscopic sphincterotomy in removal of difficult common bile duct stones: a literature review. World J Gastroenterol. 2014;20(24):7760-7766. doi.10.3748/wjg.v20.i24.7760.