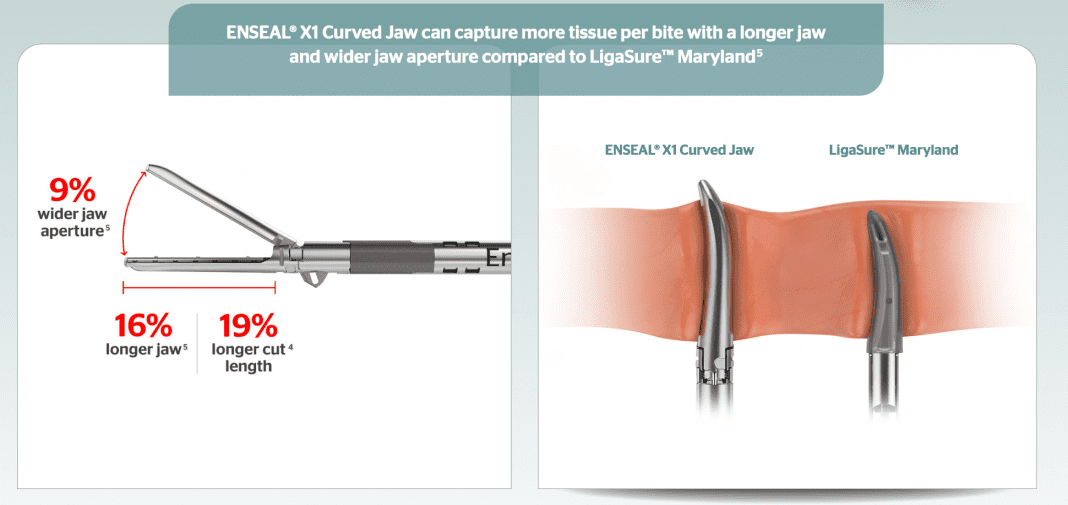
Ethicon*, part of the Johnson & Johnson Medical Devices Companies,** today announced the launch of the ENSEAL X1 Curved Jaw Tissue Sealer, a new advanced bipolar energy device that increases procedural efficiency1 and provided stronger sealing2 and better access to more tissue than LigaSure™ Maryland.3
The device is indicated for colorectal, gynecological, bariatric surgery and thoracic procedures.
The ENSEAL X1 Curved Jaw is the first of several new advanced laparoscopic bipolar devices the company plans to launch in the coming months as it expands its extensive energy portfolio, which includes market-leading HARMONIC ultrasonic devices and MEGADYNE core electrosurgical tools.
Among the ENSEAL X1 Curved Jaw’s new features are separate seal and cut capabilities, a 360-degree continuous shaft rotation that enables easy access to targeted tissue,4 and Ethicon’s Adaptive Tissue Technology, which enables the device to continuously sense changes in the condition of tissue and respond accordingly with the optimal amount of energy to minimize lateral thermal spread.5,6 These features, including improved ergonomics and a one-handed operation, combine to offer precision, a secure seal and a more intuitive and simplified use that may deliver greater efficiency in the operating room.7,8,9,10
“This is an intelligent and intuitive energy device that provides secure sealing and an ease of use that improves upon currently available advanced bipolar sealing devices,” said Steven McCarus, MD, Chief of GYN Surgery at AdventHealth Celebration.*** “It feels great in your hand, and I think it will make a real difference in procedures in terms of patient outcomes and procedural efficiency.”
Ethicon previously launched the ENSEAL X1 Large Jaw Tissue Sealer for vessel sealing in open surgical procedures and to provide better hemostasis, minimize thermal spread, and improve ergonomics than Medtronic’s LigaSure Impact.11,12,13
“Ethicon is the only company with the expertise deep enough and the energy portfolio broad enough to offer tailored, proven and reliable product and service solutions that span virtually every energy modality, surgical specialty and procedure,” said Kate Masschelein, President, Ethicon. “We will continue to develop innovative solutions that leverage our decades of experience in surgery and our deep understanding of the science of tissue management, device-tissue interaction and OR safety to set new standards of performance in surgery.”
*Ethicon represents the products and services of Ethicon, Inc., Ethicon Endo-Surgery, LLC and certain of their affiliates. All other trademarks are the property of their respective owners.
*Ethicon represents the products and services of Ethicon, Inc., Ethicon Endo-Surgery, LLC and certain of their affiliates. All other trademarks are the property of their respective owners.
**The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopedics, and interventional solutions businesses within Johnson & Johnson’s Medical Devices segment.
***Consultant to Ethicon