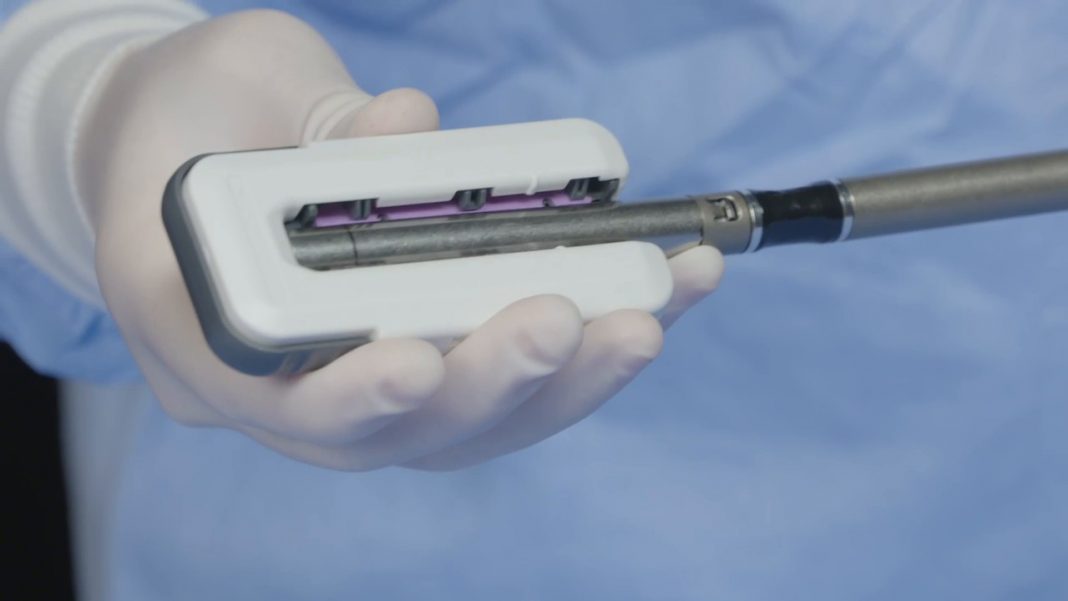
Ethicon, part of Johnson & Johnson Medical Devices Companies, announced today the launch of ECHELON ENDOPATH Staple Line Reinforcement (SLR), a novel buttressing device designed to further strengthen staple lines and reduce potential complications during bariatric, thoracic and general surgical procedures. This is the company’s first buttress solution designed for use with its own industry-leading ECHELON FLEX™ Powered Staplers with GST reloads, which have been associated with a lower rate of air leaks and bleeding complications.[i],[ii]
Ethicon developed the ECHELON ENDOPATH SLR to help surgeons who want even more strength around the staple line and to overcome issues with current buttressing options which can be difficult to load, result in inconsistent staple line coverage and may loosen with typical maneuvering during stapling procedures.
“The ECHELON ENDOPATH SLR takes a different approach to buttressing that offers significant improvements in ease of use, functionality and OR efficiency.[iii] For those who buttress, I expect this will become their preferred solution,” said Neil Floch MD, FACS, Director of Minimally Invasive and Bariatric Surgery, Norwalk Hospital.***
Designed for ease of use, the ECHELON ENDOPATH SLR delivers a seamless buttress experience from start to finish.[iv] The device’s simple click-and-go applicator and proprietary buttress attachment material are designed for precise control, consistent and quicker loading, and a more secure attachment for uninterrupted tissue manipulation and release.[v] The new device prevents the slipping, twisting, sliding or bunching that can occur with other buttress options and provides greater stability when manipulating tissue.[vi]
The ECHELON ENDOPATH SLR also provides exceptional coverage after tissue manipulation. Studies show 100% of its applications covered all staples with buttress[vii] compared to 28%[viii] for GORE® SEAMGUARD® Bioabsorbable SLR and 0% for Medtronic’s Endo GIA™ Reinforced Reload with Tri-Staple Technology.[ix] The Ethicon device also showed higher average reinforcement,[x] and takes fewer steps and less time to load than the GORE product.[xi]
“The launch of the ECHELON ENDOPATH SLR marks a new milestone in Ethicon’s continuing commitment to provide a better way to staple, and now a better way to buttress, when needed in minimally-invasive surgical procedures,” said Tom O’Brien, Worldwide President Endomechanical, Ethicon, Inc. “At Ethicon, we continually look for ways to optimize usability for surgeons and improve patient outcomes.”
[i] Miller DL, Roy S, Kassis ES. et al. Impact of powered and tissue-specific endoscopic stapling technology on clinical and economic outcomes of video-assisted thoracic surgery lobectomy procedures: a retrospective, observational study. Advances in Therapy (2018). https://doi.org/10.1007/s12325-018-0679-z.
[ii] Klassen C, Eckert CE, Wong J. et al. Ex Vivo Modeling of Perioperative Air Leaks in Porcine Lungs. IEEE Transactions on Biomedical Engineering (2018). https://ieeexplore.ieee.org/document/8325303/
[iii] Wong JB, Henninger DD, Clymer JW. et al. A Novel, Easy-to-Use Staple Line Reinforcement for Surgical Staplers. Medical Devices: Evidence and Research. 2020:12 23-29.
[iv] Based on coverage after manipulation assessment on porcine tissue.
[v] Based on coverage after manipulation assessment on porcine tissue.
[vi] Based on coverage after manipulation assessment on porcine tissue.
[vii] In a tissue test ECHELON ENDOPATH™ Reinforcement covered all staple legs in (40/40) applications compared to GORE™ SEAMGUARD™ (11/40), p<0.05.
[viii] In a tissue test ECHELON ENDOPATH™ Reinforcement covered all staple legs in (40/40) applications compared to GORE™ SEAMGUARD™ (11/40), p<0.05.
[ix] In a tissue test ECHELON ENDOPATH™ Staple Line Reinforcement fully covered staple legs in (15/15) applications compared to Endo GIA™ Reinforced Reload with Tri-Staple™ Technology (0/15), p<0.05.
[x] Reinforcement measured via in vitro staple pull-through force at T=0, T=7, and T=14 days resulting in mean values: ECHELON ENDOPATH™ Reinforcement (22.02N, 10.65N, 2.93N) vs. GORE® SEAMGUARD® Reinforcement (7.33N, 5.52N, 2.35N), p<0.05. 109221-190311
[xi] Ricketts C, Pollack E. Evaluation of setup by nurses of a novel click and go staple line reinforcement system. Glob Surg. 2020;6. doi:10.15761/GOS.1000215.