Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced today that it has been granted patent #11,490,966 by the United States Patent and Trademark Office.
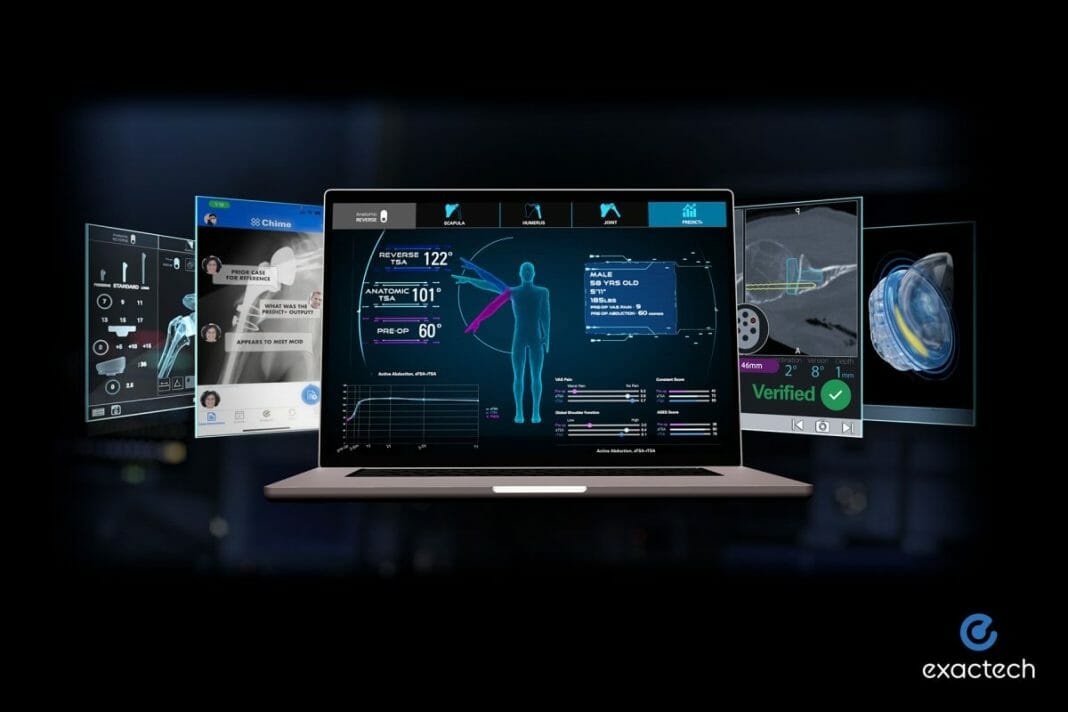
This newly issued patent describes a method and system to predict outcomes following arthroplasty procedures and relates to Predict+. Predict+ is a data-driven, clinical decision support tool and the first software of its kind to use machine learning to predict individual patient outcomes after shoulder replacement surgery. The software was released in November 2020 and was designed to better inform surgeons regarding the expected outcomes that can be achieved after shoulder arthroplasty, based on the clinical experience documented within the world’s largest single-shoulder prosthesis outcomes database, consisting of more than 15,000 patients.
“Predict+ is a novel application of orthopedic clinical research and data science and represents a significant, value-creating advancement to the surgeon-patient preoperative consultation process related to shoulder arthroplasty,” said Chris Roche, Exactech’s Senior Vice President of Extremities. “This granted patent strengthens Exactech’s leadership position in the orthopedic applications of artificial intelligence and features several broad claims which cover future innovations and expands Exactech’s intellectual property portfolio.”
With Predict+, the surgeon inputs as few as 19 data points about a patient and within seconds, the software predicts the patient’s potential outcomes, including pain, function, and range of motion. In addition, it compares predictive results for anatomic and reverse shoulder arthroplasty at multiple post-operative timepoints. This guidance can help the surgeon better personalize patient treatment by identifying factors that drive the outcome predictions, including modifiable factors such as the patient losing weight, quitting smoking, and completing pre-habilitation. Finally, Predict+ aggregates the outcomes and complications within the database so that surgeons and patients can compare their personalized predictions with the clinical experience of patients of similar age and gender after anatomic and reverse shoulder replacement.
“Developed in partnership with Advata, Predict+ showcases the predictive power of machine learning to transform healthcare,” said Vikas Kumar, Advata’s Vice President of Research and Machine Learning. “Predict+ delivers personalized, evidence-based predictions that objectively quantify the risk and benefit that an individual patient may experience after anatomic and reverse shoulder replacement, aligning patient and surgeon expectations for shared decision making.”
Predict+ is built upon previously published, peer-reviewed research and is the latest in a fast-growing line-up of technologies that power Exactech’s Active Intelligence platform. Predict+ supports Exactech’s Equinoxe® shoulder, the industry’s fastest growing and most studied shoulder system. It is accessible through the Equinoxe Planning App preoperative planning software, which can be used with the ExactechGPS® navigation system in the operating room, connecting the preoperative plan with implant placement.
Predict+ is available to surgeons globally on a limited basis. Please contact your Exactech representative for additional information.