Explorer Surgical, a GHX company, has expanded its presence in the pediatric orthopedic medical device space with the announcement of its newest customer, Pega Medical. Specializing in the design, development, evaluation and manufacturing of medical devices for pediatric orthopedics, Pega Medical was founded in 1996 and its implantable devices reach children living with orthopedic conditions in more than 70 countries, including within multiple top-tier children’s hospitals.
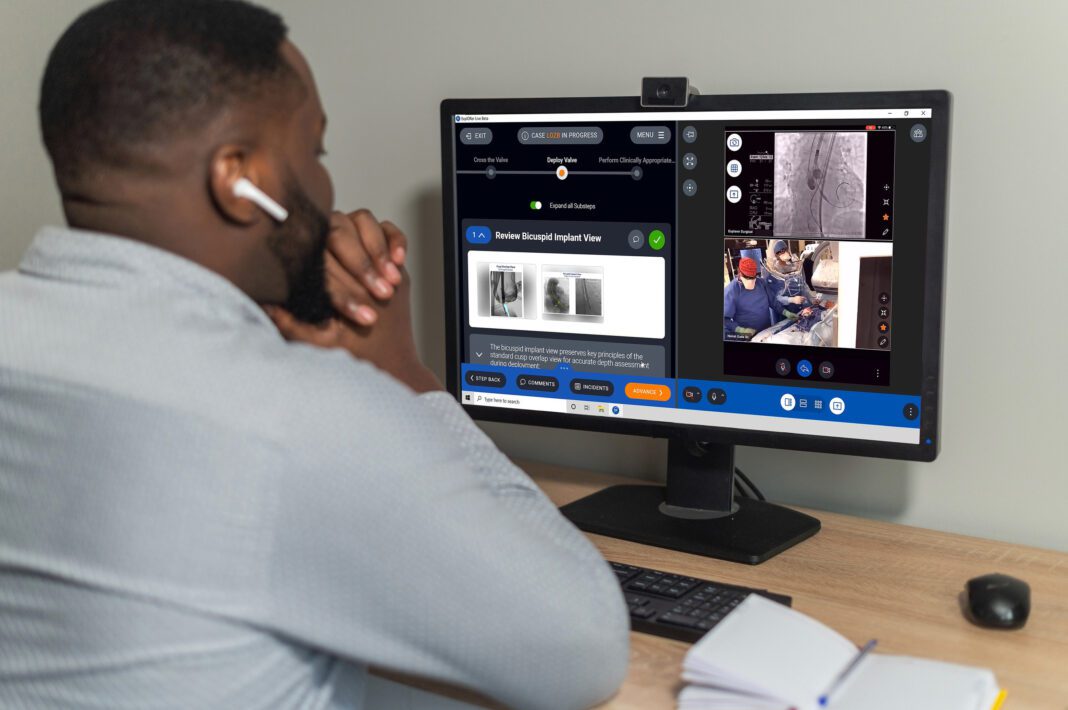
Pega Medical’s medical device reps and senior-level clinical teams will now have access to Explorer Surgical’s digital case support platform that facilitates sharing procedural best practices remotely and real-time collaboration during live procedures.
Explorer Surgical’s innovative procedural playbooks, telementoring and performance tracking tools help providers and medical device companies better collaborate and drive procedural efficiencies and repeatable processes to enhance patient outcomes. In fact, a recent study from the Journal of Pediatric Orthopaedics cites “…the COVID-19 pandemic and its related countermeasures have had significant impacts on pediatric orthopaedic practice and increased uptake of technology to provide care continuity.” The impact the pandemic has had on this specialty demonstrates the immense need for technological integrations that can make procedures more efficient and teachable to give children optimal care and better outcomes.
“We believe the Explorer Surgical platform will modernize the way our clinical and field sales teams train and support surgeons and OR staff,” said Pega Medical VP of Product Development and Marketing Fady Rayes. “With clinical personnel located all over the world, the Explorer Surgical platform gives us the ability to connect and collaborate remotely and in real-time, providing our team with greater flexibility and efficiency, particularly when we are not on-site for a live case. Our primary focus is on the outcomes for the children receiving our implants and Explorer Surgical will be a tremendous asset in advancing our work.”
“Extending the highest quality care and the best possible outcome to all patients is at the heart of our mission, but it’s especially important for children as they are one of the most vulnerable patient populations,” said Explorer Surgical General Manager and Co-founder Jennifer Fried. “As a parent myself, there are few things as rewarding as helping a child live a fuller, healthier life. We are honored to help Pega Medical in its quest to put state-of-the-art technologies in the hands of surgeons who understand the unique complexities in caring for children.”
To learn more, join Pega Medical at booth #3663 (South Building) and Explorer Surgical at booth #118 (South Building) at the 2022 American Academy of Orthopedic Surgeons (AAOS) Annual Meeting, taking place March 22-26 at the McCormick Place in Chicago.