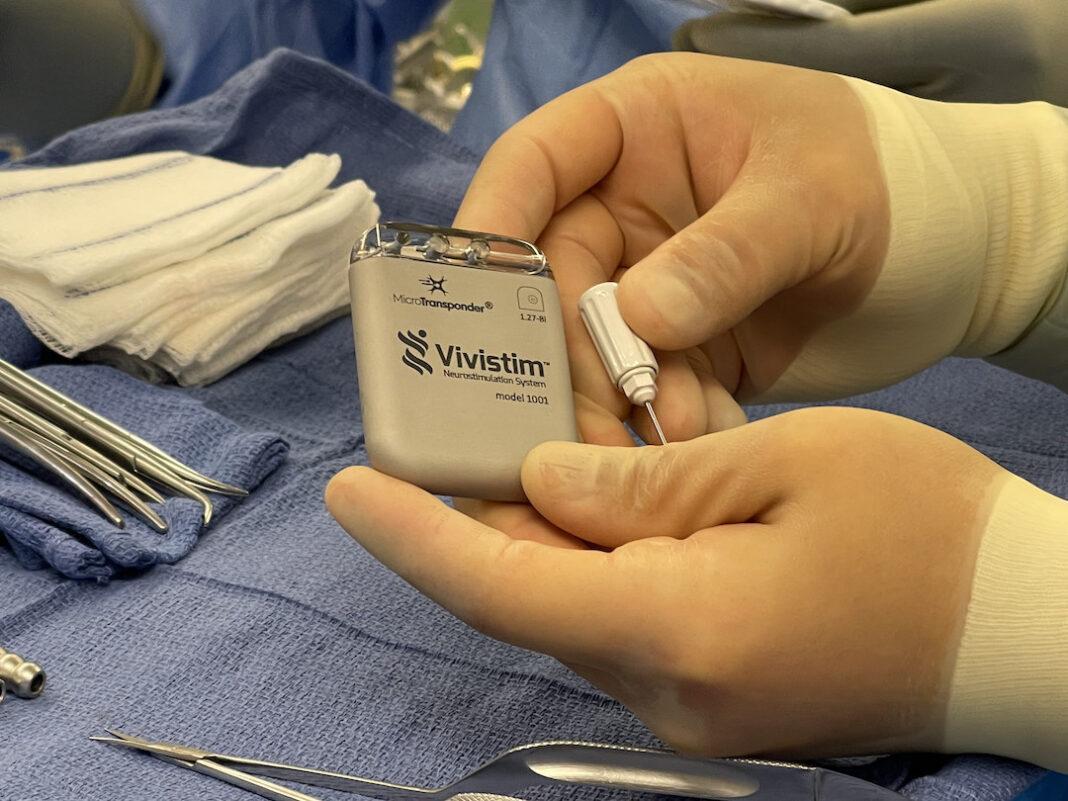
VNS Therapy
MicroTransponder®, Inc. reached a milestone Tuesday with the first commercial use of its Vivistim® Paired VNS™ System. A medical device company that develops solutions to restore independence and dignity for people suffering from neurological conditions that impair sensory and motor function, MicroTransponder announced that a surgical team, Charles Y. Liu, MD, Ph.D., director of the USC Neurorestoration Center and Jonathan J. Russin, MD, director of the USC Neuro Revascularization Center, from Keck Medicine of USC, successfully placed the small Vivistim device under the skin of Rosa Maria Villalpando’s upper left chest area during an outpatient procedure.
“We are proud that the Vivistim System is reviving hope for stroke survivors and addressing an unmet need for those who have chronic impairment,” said Richard Foust, MicroTransponder’s CEO. “Stroke survivors whose hand and arm function have not significantly improved can now collaborate with their healthcare professionals to assess if they are potential candidates for Vivistim.” When used in conjunction with rehabilitation therapy, the Vivistim System employs paired vagus nerve stimulation (VNS) to generate two to three times more hand and arm function improvement for stroke survivors than rehabilitation therapy alone1.
During rehabilitation therapy, a therapist will signal the device to deliver a gentle pulse to the vagus nerve while the stroke survivor performs a specific or functional task, such as cutting food, buttoning a shirt, or playing games. This pairing of the rehabilitation exercise with VNS releases neuromodulators that create or strengthen neural connections in areas of the brain outside the damaged region, improving upper limb function and increasing the effectiveness of the therapy.
Stroke Survivor Chooses Paired VNS Therapy for Rehabilitation
Villalpando, a clinical psychologist and former news reporter and anchor for the Los Angeles Univision affiliate, reached a plateau in her physical therapy in 2019, having only limited use of her hand and arm. Eager to regain her independence to cook, clean and style her hair, Villalpando talked to another stroke survivor, Elaine Turner, who, as an early user of Vivistim during the clinical trials, is now able to lift her affected arm shoulder-height and use her affected hand to roll down the windows and press the turn signal in her truck.
“That was the deciding factor for me,” said Villalpando, whose sister has been helping her with daily tasks and activities. “I’ve tried everything and I miss being able to use my hand and arm. I was motivated after speaking to Elaine about her hand and arm recovery and hearing how Vivistim enabled her to get back to doing the things she loved.”
Before her stroke five years ago, Turner says she was “living her best life,” riding her motorcycle and bicycle, going to aerobics classes and line dancing while balancing work as a compliance analyst and responsibilities as a mother and grandmother. Now retired, she gave Vivistim Therapy a try because she was adamant that she would be able to do everything she could before the stroke, even if it took longer.
“’No’ has never been acceptable for me, so when I was presented with Vivistim, I knew I had to take advantage of this resource and give it my full effort,” said Turner, who engaged in Paired VNS Therapy at Rancho Los Amigos National Rehabilitation Center’s Rancho Research Institute and is back on her bicycle, driving and doing her daily household activities. “Physical therapy is hard, but one day when I realized I could get out of bed by pushing up on my affected arm, it clicked for me that Vivistim is working by connecting my brain to my hand and arm, allowing me to be independent again.”
Paradigm Shift in Neurorehabilitation begins with Vivistim
Liu, who was also lead neurosurgeon of the Vivistim clinical trial at Rancho Research Institute, utilized paired VNS for both Turner and Villalpando.
“Vagus nerve stimulation paired with rehabilitation represents an exciting improvement in neurorestoration after stroke,” said Liu. This is the first time vagus nerve stimulation in conjunction with rehabilitation will be utilized for post-stroke treatment with the goal of restoring stroke survivors’ upper extremity mobility.”
Once Liu and Russin clear Villalpando for physical activity, she will begin her Paired VNS Therapy journey with the Keck Medicine rehabilitation team led by Ramzi Ben Youssef, MD, medical director of the acute rehabilitation unit at Keck Hospital of USC.
“Clinical trial results have shown that stroke survivors may benefit from vagus nerve stimulation paired with rehab,” said Ben Youssef. “By stimulating the vagus nerve, we’re hoping to make a change in the microenvironment of the brain, creating or strengthening neural connections that bypass stroke-damaged areas in the brain and enhance the relevance of physical therapy.”
Restoring Independence with Proven Outcomes
Every year approximately 800,000 people in the United States have a stroke and up to 60% of these survivors suffer from persistent impaired upper limb function six months after the stroke.
The Vivistim System received FDA approval following a decade of preclinical research and a 108-person, multi-center, triple-blinded, randomized controlled clinical trial that was peer-reviewed and published in The Lancet, an esteemed medical journal. Stroke survivors who have benefited from Vivistim reported improvement across numerous quality of life measures, including functional mobility, self-care and daily living.
Additional Vivistim case starts are expected in the coming months at leading comprehensive stroke centers, as more clinical teams across the country identify potential candidates for the Vivistim System. Stroke survivors can ask their rehabilitation specialist or physician if they are candidates for Paired VNS therapy. They would then need to be referred to a comprehensive stroke center offering Vivistim.
Ready to learn more about Vivistim? Click here to connect with the MicroTransponder team.