GE Healthcare and Optellum today announced that they have signed a letter of intent to collaborate to advance precision diagnosis and treatment of lung cancer. GE Healthcare is a global leader in medical imaging solutions. Optellum is the leader in AI decision support for the early diagnosis and optimal treatment of lung cancer.
Together, the companies are seeking to address one of the largest challenges in the diagnosis of lung cancer, helping providers to determine the malignancy of a lung nodule: a suspicious lesion that may be benign or cancerous. The majority of incidentally detected pulmonary nodules present an indeterminate cancer risk, and are incredibly challenging for clinicians to diagnose and manage, leading to delayed treatment for cancer patients and invasive procedures on healthy people.
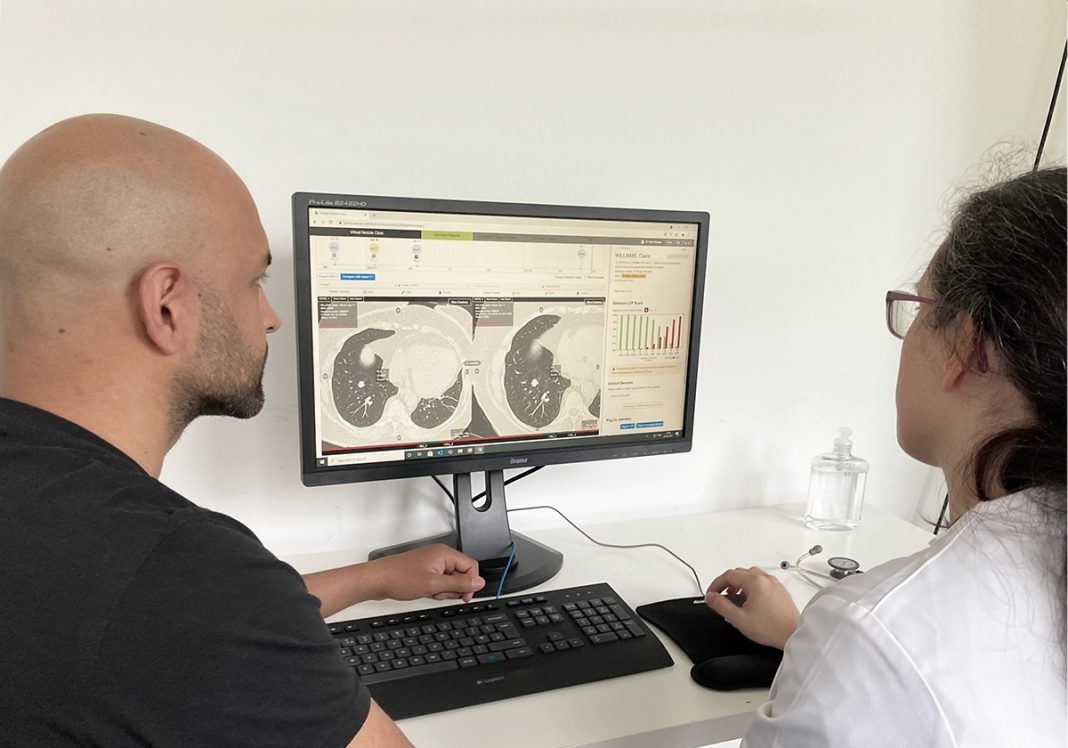
Optellum’s Virtual Nodule Clinic identifies and scores the probability of malignancy in a lung nodule, which is key to determining whether biopsy is necessary, and accelerating diagnosis. It is the only FDA-cleared AI-assisted diagnosis software for early-stage lung cancer1, and has been shown to improve the sensitivity and specificity of malignancy assessments of indeterminate nodules 2 — enabling pulmonologists and radiologists to make optimal clinical decisions3.
The clinician’s AI-assisted diagnosis of malignancy may enable patients whose nodules are not malignant to avoid unnecessary and aggressive procedures such as biopsy and surgical resection, while expediting the diagnostic process, and enabling the right treatment to start earlier. This has the potential to provide patients with personalized diagnosis and treatment plans, enabling lung cancer patients to be treated at the earliest possible stage when survival rates are the highest.
GE plans to collaborate with Optellum’s sales team on the distribution of the Virtual Nodule Clinic and work with Optellum to integrate the platform with AI solutions powered by GE Healthcare’s Edison platform. In addition the companies intend to bring results from Optellum’s Lung Cancer Prediction AI into the existing workflow of various GE Healthcare technological pathways, including CT and PACS.
“The precise diagnosis of lung cancer can greatly improve patient prognosis,” said Ben Newton, General Manager, Oncology Solutions, at GE Healthcare. “The integration of imaging and medical device data from the Edison Platform with AI-enabled solutions like the one offered by the Optellum Virtual Nodule Clinic has the potential to streamline clinician workflows and advance our goal of making precision healthcare, taking the right action at the right time for every patient, at scale, as widely accessible as possible.”
“This collaboration is a major step forward for Optellum and the field of thoracic oncology at large,” commented Václav Potěšil, Ph.D., Founder and Chief Business Officer of Optellum. “GE’s vast clinical network can accelerate deployment of Optellum’s platform and could enable a revolutionary redefinition of early lung cancer treatment for clinicians and patients around the world.”