Genomadix today announced it has entered into a know-how license agreement and stock purchase agreement with Mayo Clinic to advance its point of care molecular analyzer technology.
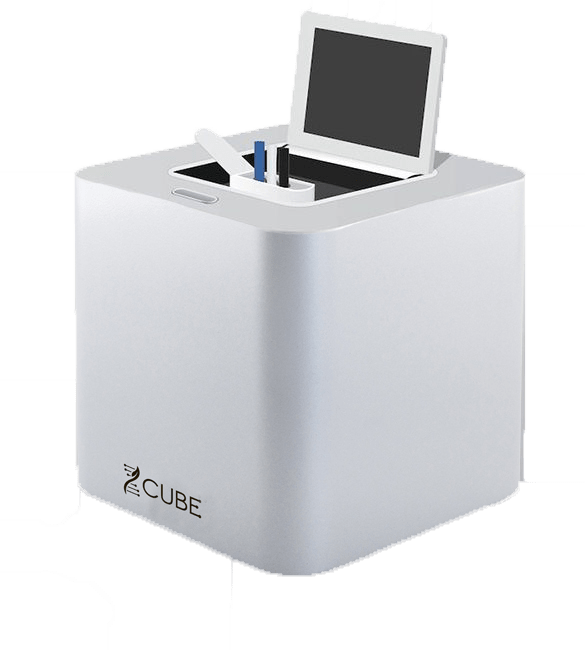
The Genomadix Cube uses polymerase chain reaction (PCR) technology in a small and portable footprint to generate timely (one hour), accurate test results, expanding the reach beyond centralized labs.
The Genomadix CubeTM testing system already delivers environmental testing for potentially deadly Legionella bacteria in water sources such as building cooling towers and CYP2C19 genotype guided antiplatelet therapy in Europe with efforts underway for US authorization. Additionally, a COVID-19 product has been developed with introduction slated (pending authorization) for later in 2022.
“We are extremely proud to be collaborating with Mayo Clinic to progress our technology and mission to make highly accurate molecular testing available for the benefit of patients. This collaboration seeks to seed new product developments with focused expertise provided by Mayo Clinic experts” commented Steve Edgett, Genomadix’s CEO
Mayo Clinic has a financial interest in the technology referenced in this press release. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education, and research.