Xenex Disinfection Services, the world leader in UV disinfection technology for healthcare facilities, today announced the 5-year renewal of its U.S. General Services Administration (GSA) contract, which enables federal agencies (including Veterans Affairs and U.S. Department of Defense healthcare facilities) to easily deploy Xenex’s powerful LightStrike™ room disinfection technology.
In addition to federal agencies, the GSA contract supports the procurement needs of eligible state, local, territorial, and tribal governments (including schools). The GSA Schedule program is the premier acquisition vehicle for the U.S. government, providing an easy and efficient way for government buyers to connect with commercial companies.
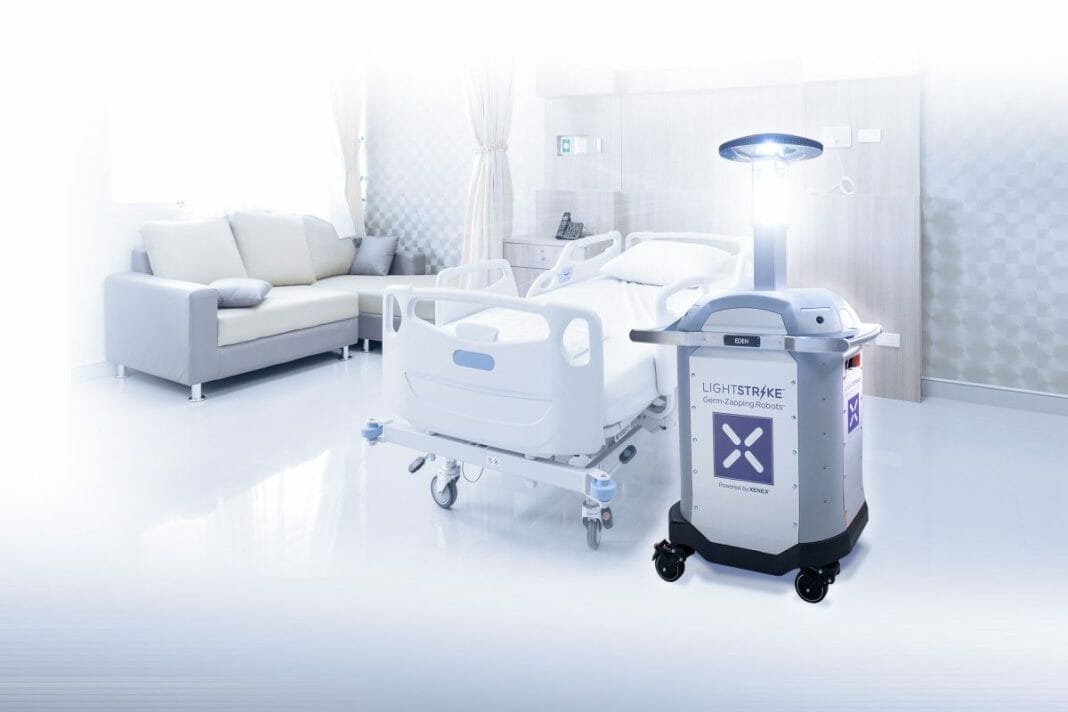
LightStrike Germ-Zapping Robots™, which first became available via GSA contract in 2012, provide a fast and effective way to disinfect healthcare facilities. More than 130 government healthcare facilities including VA, Army, Navy, Air Force, and Marine Corps hospitals use Xenex LightStrike robots for daily room disinfection. In 2012, the W. G. (Bill) Hefner VA Medical Center in Salisbury, NC was the first VA hospital to deploy four LightStrike robots and today, utilizes its 11 LightStrike robots to disinfect ORs, ICUs, patient rooms, the Emergency Department, catheterization lab, oncology, dialysis, public restrooms and much more.
Why is disinfection necessary?
Studies show that less than half the surfaces in a patient room are disinfected when it’s being cleaned and prepared for the next patient. Pathogens such as Clostridium difficile (C.diff), methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter, and Candida auris that may be left behind on high touch surfaces can transmit from patient to patient or patient to healthcare worker. Some deadly superbugs are showing resistance to cleaning chemicals, making the pathogens even more difficult to remove.
The LightStrike robot utilizes intense bursts of pulsed xenon ultraviolet (UV) light to quickly deactivate viruses, bacteria and spores on surfaces and is effective against even the most dangerous superbugs and multi-drug resistant organisms, including MRSA, C.diff, and SARS-CoV-2 (the virus that causes COVID-19). Additionally, the LightStrike robot has been proven effective against both the Ebola virus and Anthrax and can easily be incorporated into a facility’s biodefense strategy.
“There’s never been a more important time to have a disinfected environment. Viruses and bacteria are becoming resistant to chemicals, antibiotics and even some hand sanitizers. We are honored to be part of the disinfection strategy for the VA and DOD hospitals using LightStrike robots for room disinfection. Protecting the environment for those who protect us every day is an honor and we are committed to helping our government customers every step of the way,” said Joe Monroe, vice president of U.S. sales for Xenex.
Scientifically-validated
More than 45 peer-reviewed studies have been published validating the efficacy of LightStrike technology. The Central Texas Veterans Health Care System is a pioneer in the utilization of UV technology for room disinfection and has conducted numerous studies about LightStrike pulsed xenon UV robots. One study published in the American Journal of Infection Control indicates that pulsed xenon-based ultraviolet light systems effectively reduce aerobic bacteria in the absence of manual disinfection.
Environmental Protection for Government Facilities
Xenex robots are designed and manufactured in the U.S. and use pulsed xenon lamps (not mercury bulbs) to generate broad spectrum UV light. The robots don’t require warm-up or cool-down time, and don’t harm surfaces or expensive hospital equipment like mercury lamp UV products. In 2009, the U.S. Department of Energy issued an Executive Order for federal agencies to become more protective of the environment in practices including the use of non-toxic or less toxic alternatives when possible where these products meet the performance requirements of the agency.
LightStrike robots are also available via Geo-Med, LLC, a Service-Disabled Veteran-Owned Small Business (SDVOSB). GeoMed provides a broad range of medical and surgical products to Veterans Health Administration medical centers and DOD military treatment facilities via its GSA Contract and ECAT Capital Equipment Contract.