GT Metabolic Solutions Inc., the global leader in magnetic compression anastomosis surgery, announced today that the U.S. Food and Drug Administration (FDA) cleared GT Metabolic’s MagDI™ System for side-to-side duodeno-ileal (DI) anastomosis.1 The MagDI™ System is the first-of-its-kind minimally invasive surgical technique to create anastomosis without foreign materials left behind.2-4
With this FDA clearance, the MagDI™ System is poised to launch a new frontier in healthcare as we continue to deliver on our visionary milestones for efficacy in magnetic surgery,” said MedTech entrepreneur Thierry Thaure, CEO and co-founder of GT Metabolic. “After completing over 100 cases in seven clinical trials across eight countries, launching in the U.S. market with this groundbreaking technology is a major progression in our journey to advance minimally invasive surgery in the bariatric space as well as in future areas.”
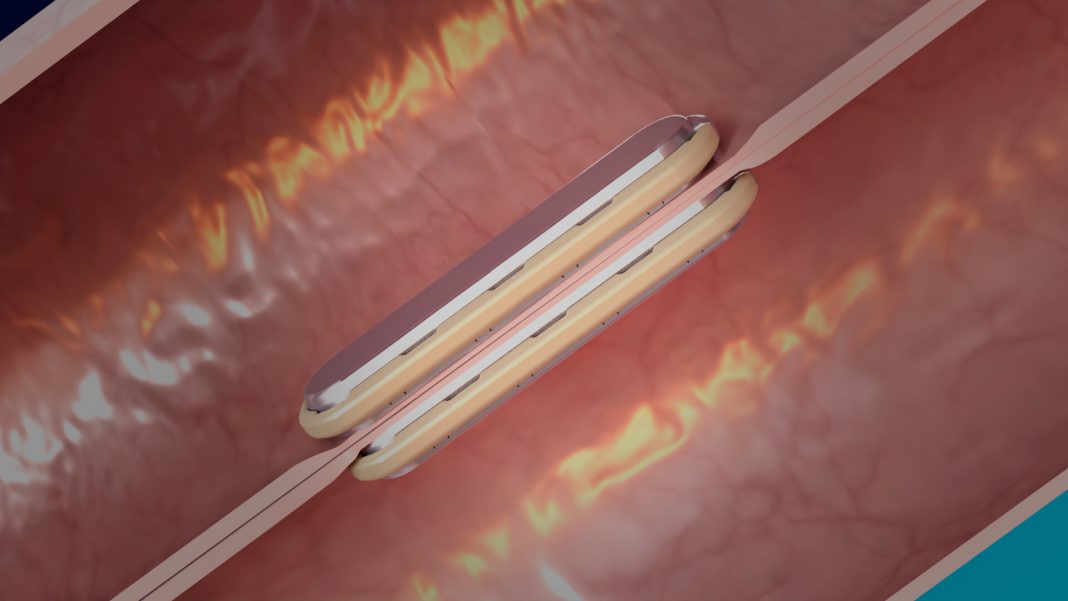
The MagDI™ System is composed of the GT Metabolic linear DI magnets, the GT Metabolic delivery system, and the GT Metabolic laparoscopic positioning device (LPD). During the MagDI procedure two linear magnets are delivered orogastrically to the patient. The magnets are positioned laparoscopically and self-align through the small bowel. After several weeks the magnets compress the tissue fusing together forming an anastomosis. The magnets detach and are expressed naturally.
The anastomosis created with magnet compressions happens without the cutting or piercing of intestinal tissue that occurs with the current practice of stapling or suturing. The MagDI™ System is designed for more consistent tissue alignment, central necrosis, and circumferential healing while leaving no foreign materials behind to impede the natural tissue healing process.2-4
The MagDI™ System was cleared after clinical data submitted to the FDA showed the system performed as intended. In all subjects, the MagDI™ System created patent side-to-side duodeno-ileal anastomosis. There were no reports of anastomotic bleeding, leakage, or obstruction.1
“It’s a paradigm shift creating a new standard of care that democratizes anastomosis creation,” said inventor Michel Gagner, MD, FRCSC, FACS, and chief medical officer and co-founder of GT Metabolic. “We’re providing the surgical community with a novel approach to minimally invasive surgery shown to have zero bleeds and zero leaks. Suturing and stapling bowel tissue for anastomosis creation will become outdated. Magnetic compression anastomosis technology will revolutionize the industry.”
Prominent U.S. bariatric surgeon Paul Enochs, MD, FACS, FASMBS, of Bariatric Specialists of the Carolinas was the first U.S. surgeon to have patients enrolled in clinical trials using the MagDI™ System. “Seeing the science and technology that’s behind this procedure has definitely opened my eyes to what the future could hold,” said Enochs.
GT Metabolic is currently identifying key sites for additional clinical studies. Key opinion leaders in the bariatric surgery specialty interested in participating in the MagDI™ System launch can contact the company at GT Metabolic.