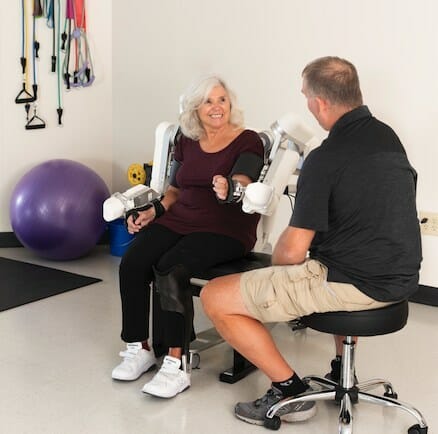
Harmonic Bionics, the robotics company for augmenting human movement, announced today that Healthlink Holdings Ltd. will be the exclusive distributor of Harmony SHR™ in the Hong Kong and Macau territories. Healthlink will begin purchasing systems following FDA registration of Harmony SHR and a full commercial launch next year.
Harmony SHR’s anatomically matched shoulder design allows a larger and more natural range of motion for patients recovering from neurological or musculoskeletal impairments compared to other upper extremity exoskeletons on the market today. Harmony was also built to help facilitate evidence-based principles in a rehab session, such as mirror therapy that has shown to improve outcomes in chronic stroke patients.
“The incidence of stroke in Hong Kong has increased over 50% in the last ten years so we know that there is a need for technology and innovation to help recovering patients,” said Healthlink Holdings Sales & Marketing Director Samuel Chan. “While we have been in the rehabilitation robotics industry for many years, we have never seen an upper extremity device like Harmony SHR that can address both neurological and orthopedic markets with its shoulder functionality and assessment capabilities, so we are excited to help make this available to patients and providers in Hong Kong and Macau.”
“While we are primarily focused on the US market at this stage, we are always looking for partnerships in territories that will help grow the adoption of our technology worldwide,” said Harmonic Bionics CEO Christopher Prentice. “With Healthlink’s existing relationships with public and private hospitals and sports clinics in Hong Kong and Macau, they will be a great ally for Harmonic Bionics.”
More here.