February 2, 2021
Hatch Medical, L.L.C. notes the exclusive brokerage relationship is with inventor and CEO, Dr. Robert Ward, MB BS FRCS FRCR, and his company, Fixit Medical, Ltd. Hatch Medical will seek to sell or out-license Dr. Ward’s next generation drainage catheter fixation device, Cingo.
Dr. Ward, a specialist in vascular and interventional radiology, has developed the most advanced and patient-centric drainage catheter fixation device available today. We are delighted to be partnering with Fixit Medical to bring their exciting technology to the market,” said Paul Gianneschi, Managing Principal of Hatch Medical.
Image-guided percutaneous drainage has become the therapeutic treatment of choice for a wide variety of abnormal fluid collections such as abscesses and ascites, and is also widely used for the relief of renal and biliary obstruction. These procedures have resulted in reduced morbidity and mortality and have helped to reduce length of hospital stays and costs. A drainage catheter is generally inserted to accomplish this clinical outcome by an interventional radiologist, a physician who specializes in image-guided procedures. The global interventional radiology drainage procedures market was valued at US$ 0.59 Bn in 2019 and is projected to expand at a CAGR of ~6% from 2020 to 2030.
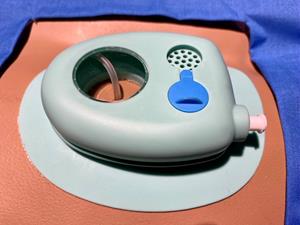
Fixit Medical’s Cingo™ is the future of catheter fixation, featuring industry leading catheter fixation, durability and functionality. The device is produced out of a soft thermoplastic elastomer in two parts; a highly flexible base able to conform to curved anatomy with an integral housing and lid. There is an aperture within the base that accepts the drainage catheter and with the lid in place the device provides complete protection to the catheter, including a water-tight seal ideal for showering.
Cingo™ features best in class catheter fixation through a pull-force dissipating design and an estimated two-week wear time. Cingo™ also boasts a revolutionary design that protects catheters from twisting and kinking, provides easy access to the catheter exit site for improved visibility and cleaning, and includes an ingenious integral shower-safe feature.
The Cingo™ drainage catheter fixation technology is available for license, sale or partnering to interested third parties through an exclusive agreement with Hatch Medical.
Dr. Ward commented, “Hatch Medical is an ideal partner given their vast experience in the interventional radiology medical device market. We look forward to working with Hatch to identify a commercial partner committed to bringing the Cingo™ drainage catheter fixation device to market, so that patients and healthcare institutions can enjoy the benefits this game changing technology brings when compared to current fixation methods.”