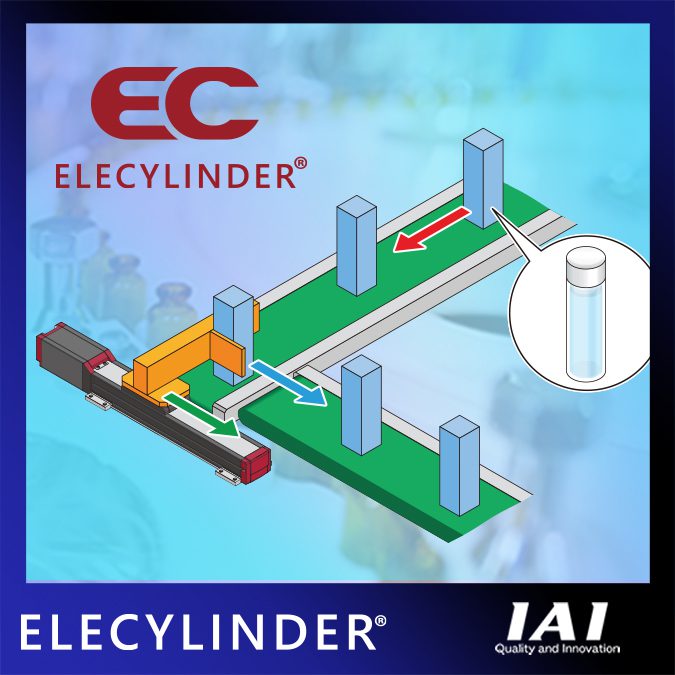
IAI (Intelligent Actuator Incorporated) would like to introduce their ELECYLINDER® electric actuator series. The main features are easy operation, AVD (acceleration/speed/deceleration) that can be adjusted individually, and high performance.
Due to its stable quality, it is ideal for the manufacturing industry of medical products where product accuracy is required.
Here is an example of how the smooth operation of ELECYLINDER® improved a packaging application. A conventional air cylinder was used to move a package containing bottles of liquid chemicals between conveyors (vertically connected). When moving at a right angle, the speed at which the product was pushed out was not constant, and the impact caused the package to fall, the liquid in the bottle to deform, or foam during movement.
ELECYLINDER® makes it easy to adjust and control AVD (A: Acceleration, V: Speed, D: Deceleration), allowing packages to move smoothly between conveyors positioned at right angles without affecting internal products. In addition, ELECYLINDER® has a wide range of products available for many types of applications.
“IAI is proud to be at the forefront of technology that supports the medical community in the fight against disease and future health crises,” said Kaz Haruna, National Sales Manager at IAI America.
IAI is actively assisting the medical community by providing Cartesian and SCARA robots for infectious disease testing, molecular diagnostics, tissue and blood sampling, genomic research, cancer research, pharmaceuticals, and medical device production. It is IAI’s hope that our ELECYLINDER® Series will help the medical/pharmaceutical industries move ahead with advanced innovation.
About IAI
Founded in Shimizu, Japan in 1976, Intelligent Actuator Incorporated (IAI) has over forty years of experience in the design and manufacture of customized medical and industrial robotics. Built on “Quality and Innovation,” IAI is a leader in the research and development of advanced energy efficient and affordable Cartesian, SCARA, and Tabletop robots. From entry-level to high-speed models, IAI provides complete programmable systems and components to fulfill any and all of your medical testing and industrial production needs.
★ USA Headquarters & Western Region
2690 West 237 Street, Torrance, CA 90505
(310) 891-6015
★ Midwest Branch Office
110 East State Parkway, Schaumburg, IL 60173
(847) 908-1400
★ Southern Branch Office
1220 Kennestone Circle, Suite 108, Marietta, GA 30066
(678) 354-9470
★ Japan Headquarters
577-1 Obane, Shimizu-ward, Shizuoka-city, Shizuoka-pref.