June 30, 2020
IlluminOss Medical, a medical device company focused on minimally invasive orthopedic fracture repair, today announced that it has received expanded U.S. Food and Drug Administration (FDA 510k) clearance for its Photodynamic Bone Stabilization System for treatment of fractures of the pelvis, clavicle, and the small bones of the hands and feet: metacarpals, metatarsals, and phalanges.
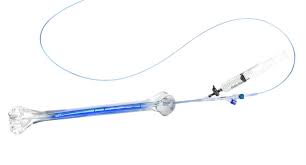
IlluminOss Medical System is a minimally invasive approach for fracture repair and stabilization through a patient-specific intramedullary implant. The system utilizes a light-curable liquid polymer, contained within an expandable balloon, to create a patient-conforming, rigid implant within the bone canal.
“The new indication expansion significantly broadens the opportunities to address the geriatric fragility fracture market where patients often have poor bone quality,” said Robert Rabiner, Chief Technology Officer of IlluminOss. “An example would be fractures of the pelvis; IlluminOss is deployed through a small, minimally invasive, tissue-sparing incision. Our system conforms to the curvature of the pelvis, and in a simple procedure provides strength and stability to the fracture. Surgeons report their patients have a rapid return to function, which is crucial for this patient population, as well as a significant reduction in the use of postoperative opioids.”
The IlluminOss technology has been in clinical use in Europe since 2010, with over 4,000 procedures to date. In the US, the IlluminOss System was previously cleared by the FDA for the treatment of traumatic, fragility, pathological, and impending pathological fractures of the humerus, radius, and ulna. IlluminOss can be also used in conjunction with FDA-cleared fracture fixation systems to provide supplemental fixation in compromised bone in all cleared anatomic sites.
“The feedback we received from the surgeons using IlluminOss in Europe described case after case of bed-ridden, elderly pelvic fracture patients up and walking the day after this very minimally invasive surgery,” said Mike Mogul, Chairman of IlluminOss. “With this indication expansion, we are excited to bring the benefits of our technology to the US patient population as well.”