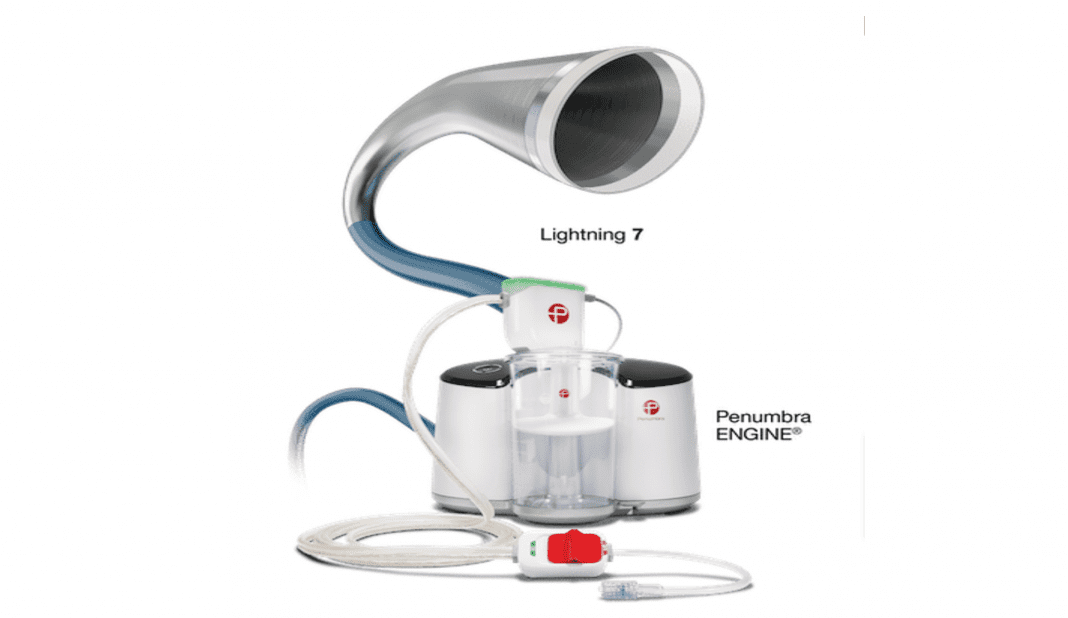
INDIGO System Lightning 7 for arterial clot removal expands Penumbra’s offering of the Indigo Aspiration System with Intelligent Aspiration for mechanical thrombectomy and is designed for single session arterial thrombus removal.
Offering a new option for physicians to address arterial thrombus removal, the Indigo System Lightning 7 combines the new Indigo System CAT™7 Aspiration Catheter with Lightning Intelligent Aspiration powered by Penumbra ENGINE®. CAT7 is a high-power, low-profile catheter that features laser-cut hypotube technology and circumferential sweep designed for dependable delivery and maximized clot extraction.
The Indigo System’s proprietary Separator™ technology is also available with Lightning 7 (Separator 7) and is designed to enable unobstructed aspiration for the duration of the procedure. Lightning Intelligent Aspiration features Penumbra’s proprietary clot detection technology that enables the operator to identify thrombus location and is also designed for blood loss reduction.
“My experience with Lightning 7 suggests that it streamlines clot removal in the peripheral arterial vasculature with low profile access, excellent trackability, and similar power to the CAT8 Penumbra catheter that was previously used for this application. The torquability makes vessel navigation much easier,” said Dr. Christopher Metzger, System Chair of Clinical Research for Ballad Health and Medical Director of Cardiac and Peripheral Cathlabs, Ballad Health, Holston Valley Medical Center, Kingsport, Tennessee. “Penumbra continues to forge ahead with yet another game-changing technology that has the potential to increase single-session thrombus removal and thereby may help improve outcomes.”
“Today in the United States, we believe the number of patients presenting to the hospital with arterial thrombus is significantly higher than patients presenting with thrombus elsewhere in the body. Conditions involving arterial thrombus are often associated with high amputation rates, and mortality as a result. We are proud to introduce another important technology to enable physicians to reduce the need for thrombolysis, which in turn can reduce demand for ICU beds,” said Adam Elsesser, president and chief executive officer, Penumbra. “We are excited by the early experience with Lightning 7 that suggests we can help physicians to simplify arterial thrombus removal with more single-session results.”
The Indigo System with Lightning is an intelligent aspiration system powered by Penumbra ENGINE and was introduced in July 2020. Lightning Aspiration Tubing has dual pressure sensors for real-time blood flow monitoring. The built-in microprocessor features a proprietary thrombus removal algorithm that automatically controls a valve in the tubing to provide continuous or intermittent aspiration. This unique mechanism of action helps optimize thrombus removal procedures by differentiating between thrombus and blood. The system is designed to aspirate continuously when in thrombus and intermittently in patent flow. When the Indigo aspiration catheter is placed at the clot and in patent flow, Lightning is designed to automatically register a change in pressure and to minimize flow with intermittent aspiration.
Throughout the case, Lightning provides procedural feedback via audiovisual cues. Lightning’s thrombus removal algorithm is designed to initiate automatic valve clicking when it senses patent flow. With automatic valve control, Lightning is designed to help reduce blood loss and allow the physician to focus on optimizing thrombus removal procedures. The Indigo System with Lightning Intelligent Aspiration is available in the United States in the following configurations: Lightning 12, Lightning 8 and Lightning 7.
The Indigo System with Lightning Intelligent Aspiration and Separators is indicated for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems, and for treatment of pulmonary embolism.