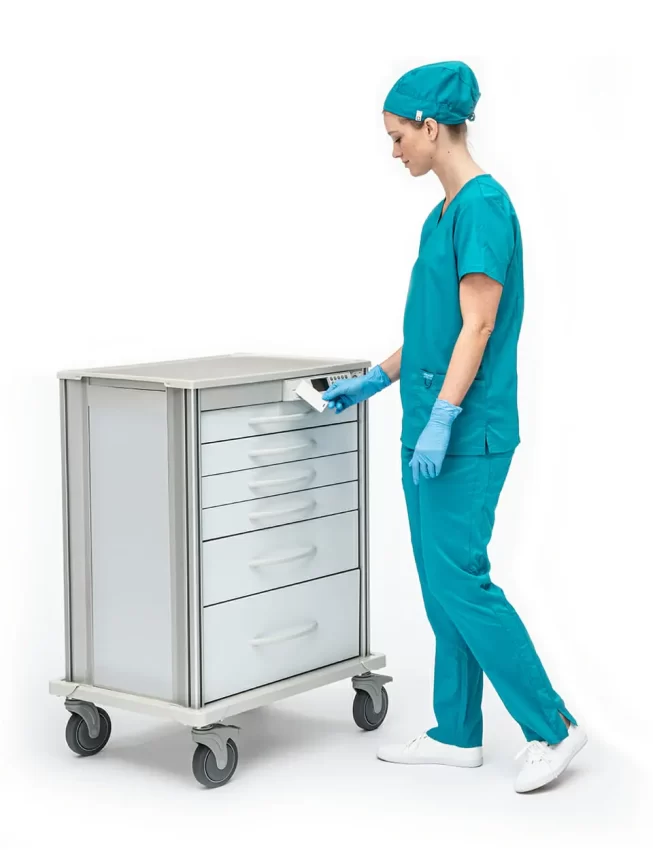
InnerSpace, a Solaire Medical Company, today announced the launch of the InterConnect Lock system.
When connected to Wi-Fi and the InnerSpace Smart Solutions cloud-based platform, InterConnect Lock Plus allows administrators to manage the security of the cart fleet remotely from anywhere. The InterConnect Lock stores up to 9,800 individual users and administrator codes per fleet and features an OLED display screen.
The InterConnect Lock has an OLED screen and keypad designed for touch responsiveness, even when users are wearing gloves. The low-gloss, textured keypad is resistant to scratching and damage from cleaning.
“InterConnect provides healthcare administrators with more information and a more secure and efficient experience,” said Ben Barber, CEO, InnerSpace. “Paired with Smart Solutions, InterConnect Plus makes it easy to change security credentials and auto-lock times as well as identify who has access to each cart. Being able to manage credentials with the stroke of a key saves time and provides added security at the cart level.”
The Wi-Fi connected InterConnect Plus allows users to submit a request to team members to facilitate cart maintenance and restocking. Automatic alerts are provided for low battery and lost network connection. A downloadable report tracks cart access, alerts and users. In the event of a power outage, data is retained and automatically uploaded when power is restored.
“The need for this type of lock product has been around for a long time, but it’s been cost prohibitive,” Barber said. “In many cases, a WiFi-enabled lock could be double the cost of a cart. That upfront cost priced past solutions out of the market. Our cloud-based subscription service offers a more affordable option that also saves administrators time managing cart fleet security.”