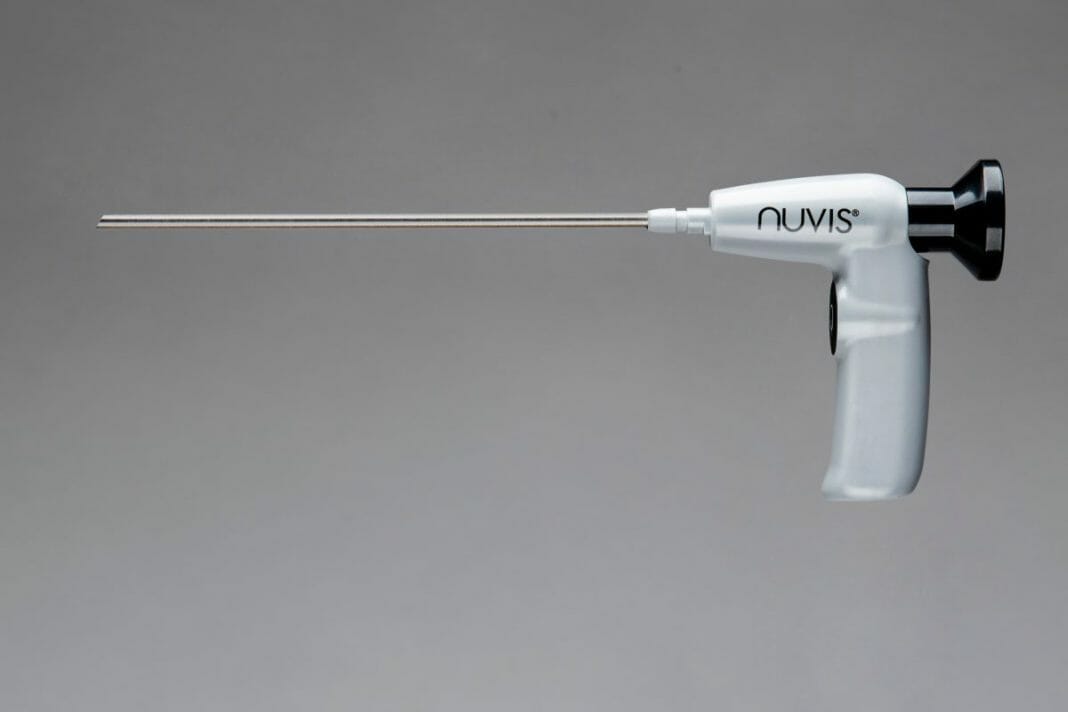
Integrated Endoscopy, a medical device company pioneering the development of 4K, low-cost, single-use endoscopes for the arthroscopic surgery market, today announced the launch of its second generation NUVIS (pronounced “nu-vee”) Single-Use Arthroscope, a first-of-its-kind 4K endoscope designed for use in arthroscopic surgical procedures.
The release of the Gen II NUVIS features a patent-pending simplified optical train design that reduces the number of optical elements by over 60% while boosting image quality to 4K. Not only is the image quality superior, but the Gen II NUVIS will also be substantially faster and less expensive to manufacture and amenable to high-volume automation. As part of the Gen II launch, Integrated Endoscopy is releasing multiple versions of NUVIS to make the technology compatible with most cannulas on the market. NUVIS is also compatible with most installed video towers and will support a 4K camera system without requiring additional capital equipment. NUVIS has a built-in battery powered LED, which eliminates the light box and bulky light cord.
“The release of the Gen II NUVIS is a huge milestone for the company in terms of image quality and compatibility, said Brad Sharp, Integrated Endoscopy’s CEO. “Our nextgen arthroscope also provides a substantial decrease in manufacturing costs while simultaneously increasing ease of manufacturability. The demand we have seen for NUVIS far exceeds our current manufacturing capacity, so the efficiencies the Gen II NUVIS affords will greatly aid in shortening production time and costs. With a global market of over 1.5 million units per month, increasing our manufacturing capacity will be a major focus for the company moving forward.”
Integrated Endoscopy’s Gen II NUVIS single-use, 4K, battery operated cordless arthroscope is FDA 501(k) cleared, CE marked, and is being registered in a number of markets around the world. As manufacturing capacity increases, the company will continue to expand its global distribution network to provide this unique technology to the arthroscopic surgical community.