March 17, 2021
Intellis Platform with Differential Target Multiplexed (DTM™) programming is used for the treatment of chronic, intractable back and leg pain.
Medtronic notes the new labeling will include study outcomes from a multicenter randomized control trial reflecting superior back pain relief with DTM SCS when compared to conventional SCS.
The trial demonstrated with clinical and statistical significance that DTM SCS programming is superior to conventional SCS programming when used to treat intractable chronic back pain. At 3 months, 80% of patients treated with DTM SCS reported back pain relief of at least 50%, compared with 51% of patients treated with conventional SCS as measured by the Visual Analog Scale (VAS), a widely used and accepted measure for pain intensity.1
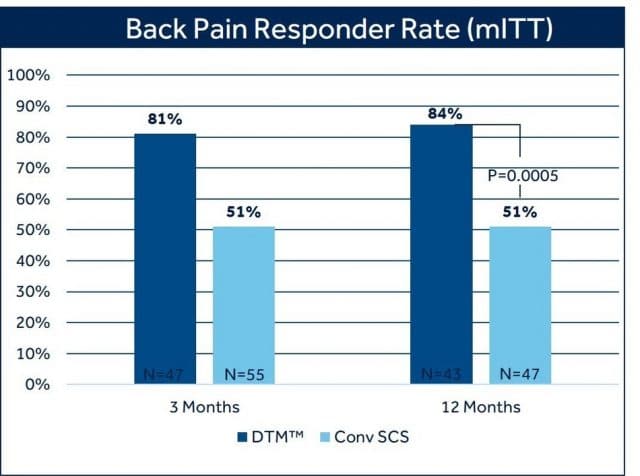
This labeling update closely follows the most recent presentation of 12-month clinical trial outcomes during a late-breaking clinical trial session at NANS 2021.2 Trial results showed statistically significant and superior back pain relief with DTM SCS compared to conventional SCS at 12 months: 84% of patients with chronic back pain treated with DTM SCS reported at least 50% pain relief, compared to 51% of patients treated with conventional SCS. There was also a difference in the proportion of patients who reported profound back pain relief (>80% reduction in VAS score) favoring DTM SCS (69%) compared with conventional SCS (35.1%).2
“The body of clinical evidence proving the efficacy of DTM SCS in treating patients with chronic back pain continues to grow,” said Charlie Covert, vice president and general manager, Pain Therapies within the Neuromodulation business, which is part of the Neuroscience Portfolio at Medtronic. “The updated labeling further strengthens the credibility of the outcomes from this therapy, and parallels the profound benefits our clinician partners are seeing with their own patients.”
DTM therapy, which is clinically proven and only available on the Medtronic Intellis platform, is a unique and proprietary programming option available to treat patients with chronic pain that is based on years of preclinical research.3,4,5
Clinical trial data can be found here. A manuscript has been submitted for consideration for peer-reviewed publication.