November 19, 2020
Caregility, a clinical collaboration and communications company moving the access point of care closer to the patient, announces the release of version 1.9 of its iObserver application, a continuous virtual observation and communications system for remotely monitoring at-risk patients.
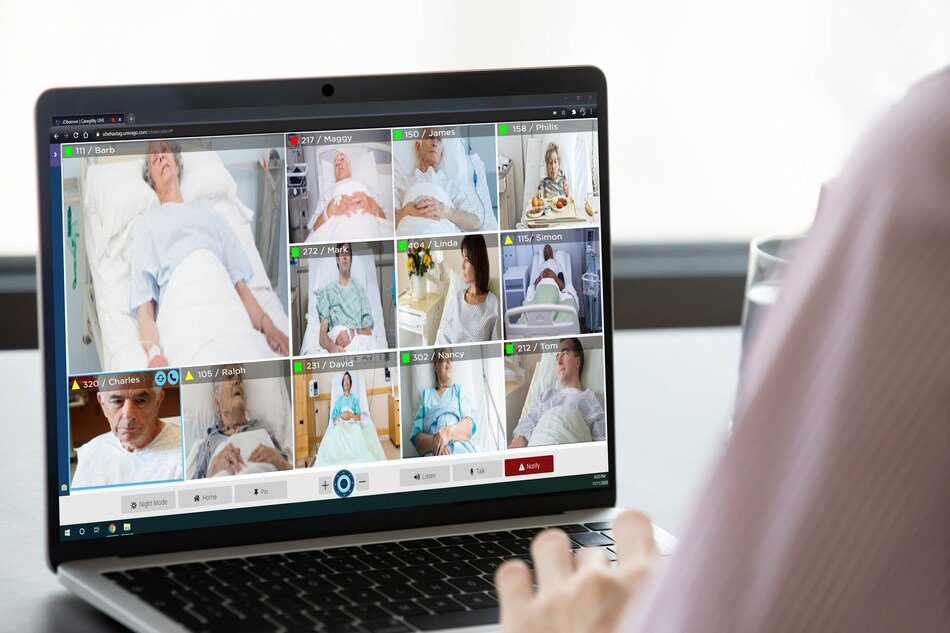
iObserver allows clinical teams to remotely observe up to 12 patient rooms on a single screen with two-way audio and video support through the Caregility Platform, a purpose-built, HIPAA-compliant telehealth ecosystem for the entire healthcare continuum.
Caregility leveraged thousands of hours of customer input to build new features and capability into the iObserver v1.9 release. The new version will enhance patient and provider experience and application flexibility with the goals of minimizing adverse events, easing provider burden, and reducing healthcare costs by enabling care teams to do more with less.
Costs associated with the patient sitting are rarely reimbursed and often unbudgeted. With expenses for in-room patient sitters running into the millions, this can have a significant impact on a hospital’s bottom-line and direct care resources. One Caregility healthcare customer, after deploying iObserver, reported that in eight months of use their unbudgeted sitter hours dropped by 75% with no falls or adverse events.
iObserver enables cost-effective virtual observation across a broad spectrum of use cases, including but not limited to:
- Clinical deterioration/rapid response events
- Fall prevention/elopement
- COVID isolation room – bedside support and room visualization
- Overflow capacity management
- Risk to self, from others, or to others
“Caregility is committed to investing in accelerated development that takes into account the experience from both the clinical perspective and the patient perspective,” said Sudhir Ahuja, SVP of Product Development at Caregility. “The iObserver v1.9 release is a great example of taking that input and turning it into a reality for our customers.”
To see a demonstration of how iObserver can help your health system protect patients and drive down costs, as well as unique options we have to help you deal with rising COVID cases with our bedside application that turns any iPad in to a secure communications device in patient rooms, contact Caregility at (732) 440-8040.