December 21, 2020
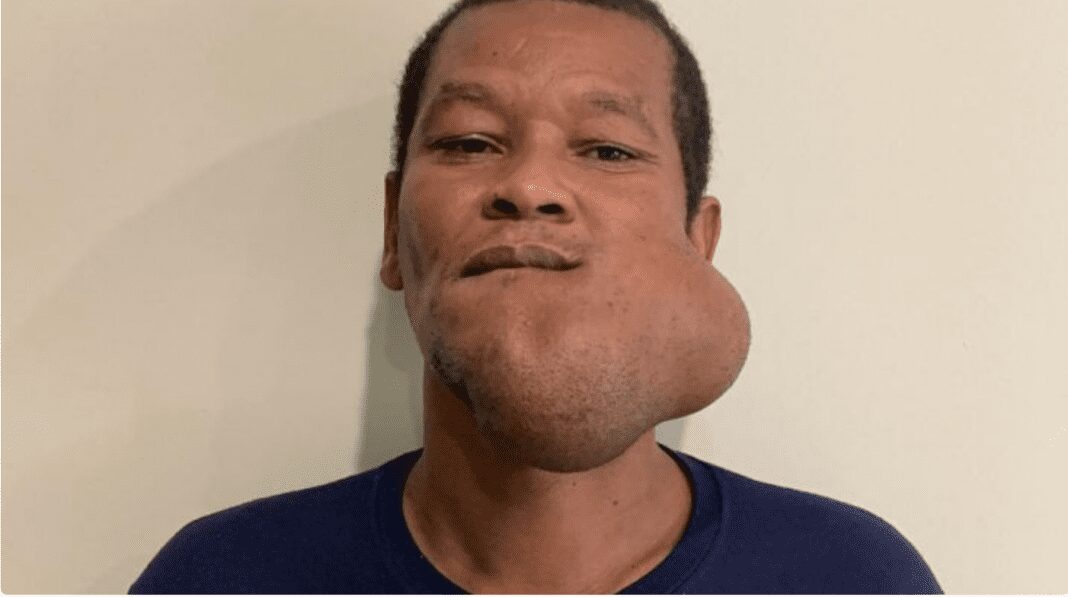
Jacob Beneby is a 46-year-old adult living with this tumor for the past 3 decades. After multiple failed surgeries, the tumor continues to grow. The tumor has him in critical pain and unable to function normally. It is estimated that this tumor weighs 10 pounds.
Jacob Beneby and his wife were flown to Florida to meet with Dr. Roland Hernandez. After reviewing his case, it was found that the tumor was growing from the inside and outside of his jaw and a series of surgeries would be needed to bring him back to health. Jaw replacement would be the only way to fully get rid of this aggressive tumor.
Jacob’s family has created a GoFundMe account in order to obtain help with the cost of medications and their stay while Jacob recovers from surgery. Their 4 children are back home in the Bahamas and can’t wait to see their parents again.
“Every day the tumor is growing. Time is of the essence” – Princess Beneby
The following procedures needed to help Jacob include:
● Once healed from his first surgery, definitive reconstructive surgery will occur in which bones from other parts of Jacob’s body will be harvested to reconstruct his face.
● The last phase will occur two years after his reconstructive surgery. Dental surgery will take place by placing dental implants where a denture can be fixated to it which will finally help Jacob Beneby achieve his dream of chewing food and having a smile again.
The family thanks Dr. Roland Hernandez, the team at Memorial Regional Hospital, and Stryker Corporation for this act of kindness and hopes that Jacob can finally smile again. To donate to their cause, please visit their GoFundMe page.