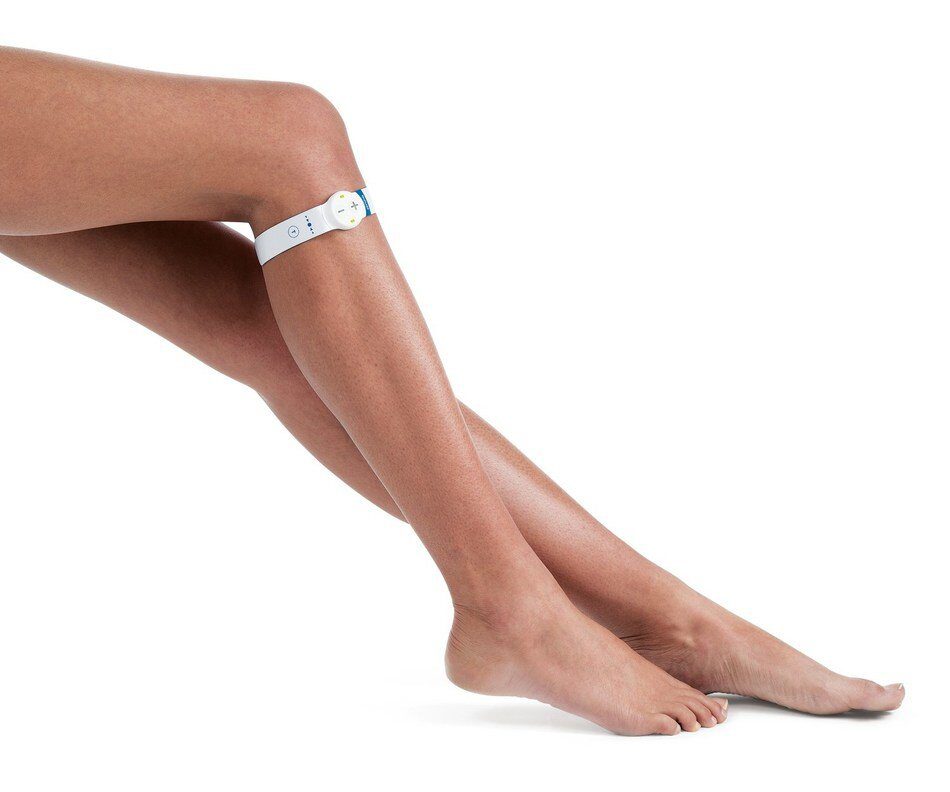
In a published study, a team from Lawson Health Research Institute has found that a simple device can reduce swelling after kidney transplantation.
The geko™ device, manufactured by Sky Medical Technology Ltd and distributed in Canada by Trudell Healthcare Solutions Inc., is a muscle pump activator that significantly improves blood flow by stimulating the body’s ‘muscle pumps.’ Patients using the device following kidney transplantation experienced shorter hospital stays and reduced surgical site infections by nearly 60 percent.
Kidney and simultaneous pancreas-kidney transplantations can significantly reduce mortality and improve the quality of life for patients with end-stage renal disease. “After surgery, many of these organ recipients require a longer hospital stay due to delayed kidney function, infection, lack of mobility or edema,” says Dr. Alp Sener, Lawson Scientist and Transplant Surgeon in the Multi-Organ Transplant Program at London Health Sciences Centre (LHSC).
Edema is swelling caused by excess fluid trapped in the body’s tissues which can impact wound healing. The current standard of care for managing lower-limb edema and improving blood flow is thrombo-embolic-deterrent (“TED”) stockings used with compression devices. Sleeves pumped with air squeeze the lower legs to boost circulation. They can be uncomfortable to wear, and the large pump can inhibit early mobility and disrupt sleep after surgery.
In a randomized controlled clinical trial spanning two years, 221 transplant recipients at LHSC either wore the standard TED stocking and pump or the geko™ device for six days after surgery. Dr. Sener’s research team found that wearing the device increased urine output by 27 percent and lowered weight gain by over a kilogram. With more urine produced and less fluid retention, patients experienced 31 percent less swelling. The duration of costly hospitalization was shortened by over one day after kidney transplantation compared to the standard of care.
A 60 percent reduction in wound infection rates was a striking observation. “Transplant patients are at a higher risk of infection due to the immunosuppressant medications needed after surgery,” explains Dr. Sener, who is also the President of the Urologic Society for Transplantation and Renal Surgery, a global organization affiliated with the American Urological Association. “Reducing infection means a much better outcome for the patient and considering that recent data shows wound infections can cost the health care system thousands of dollars per person, it’s a win-win situation.”
Some of the study participants wore pedometers to track their steps, and those using the geko™ device had improved mobility after surgery. The team suspects this may be due to reduced swelling which could improve ease and comfort when moving.
“The study results have been both surprising and exciting. Not only have we cut down wound infection rates but we have also seen a considerable improvement in the new organ’s function following transplantation. Patients report feeling more satisfied with the transplant process and are more mobile,” says Dr. Sener. The geko™ device is now being offered to patients at LHSC in recovery after receiving a new kidney.
Ruben Garcia, 68 years old, recently received a new kidney from his daughter, Ruby, who was a match as a living kidney donor. Following his surgery, Garcia found it difficult to get out of bed due to the pain and swelling, and the function of his new kidney was very low. “My surgeon explained in very simple terms that it was as if my new kidney wasn’t awake yet,” describes Garcia.
Dr. Sener recommended that Garcia use the geko™ device to help stimulate blood flow in a way that is similar to walking. Garcia was soon able to sit up on a chair and by the next day he was walking. “My kidney woke up and starting working again! I could feel the device working and it was comfortable to wear, almost like a massage for my legs. I’m very grateful for the care that I received.”
Dr. Sener adds that “using a muscle pump activator could be a game changer for other procedures like orthopedic implants where wound infection can have disastrous consequences or in surgeries where wound infections are more common such as in cancer and intestinal surgery.”
The geko™ device is non-invasive, self-adhering, battery-powered and recyclable. It generates neuromuscular electro-stimulation and unparalleled systemic blood flow that equates to 60 per cent of that achieved by walking. Pain-free muscle contraction compresses deep veins in the lower legs to create better blood flow in these vessels and return blood to the heart. It is particularly well suited to hospital settings as it portable and requires minimal training. For the indications for the use of the geko™ device, visit here.
“The results of the study provide further evidence that the geko™ device is an effective treatment option that can improve outcomes for patients and help them return home sooner, while reducing costs for the health-care system,” says George Baran, Executive Chairman of the Trudell Medical Group and a Director of Sky Medical.