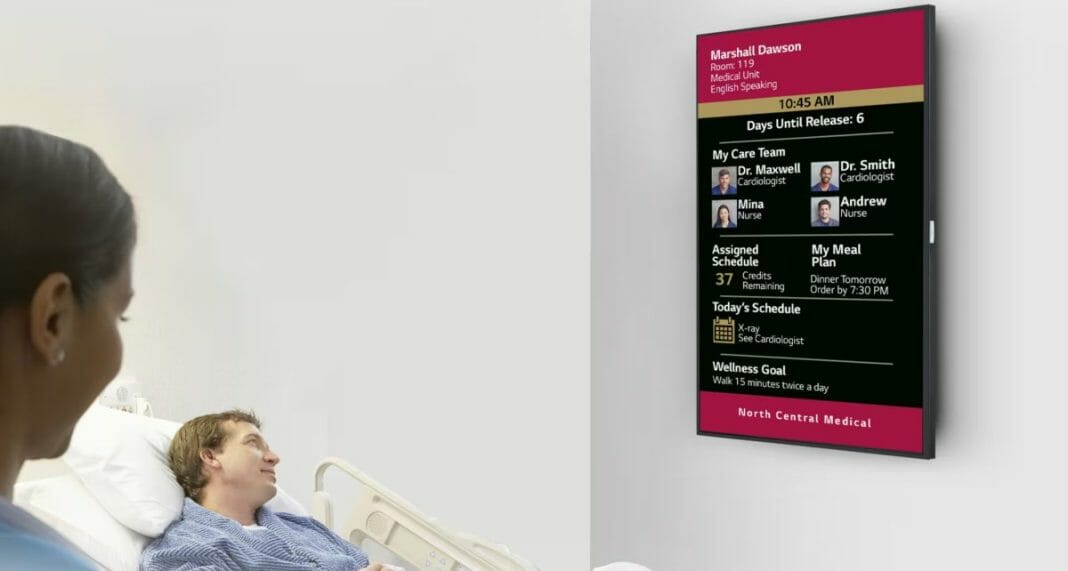
LG Business Solutions USA has launched a new line of “Patient Engagement Boards” that empower hospitals to outfit patient rooms with crisp high-definition screens for displaying patient information such as their name, schedule, a list of caregivers, native language and more. Available now, the 43-inch UHD model and 32-inch FHD model are UL-listed for Health Care and feature Power over Ethernet (PoE) capability, offering optimized solutions and simplified installation for diverse patient room needs.
“As hospitals continue to digitize more and more of their operations, in-room displays provide patients and caregivers with greater legibility and infinitely more flexibility than analog white boards,” said Tom Mottlau, LG Business Solutions USA Healthcare Director. “Our patient engagement development partners have utilized the powerful LG webOS 6.0 platform to develop intuitive applications that can allow hospitals to eliminate handwritten notes and the difficulties that can arise from illegible writing or unclear instructions. These enhancements can improve communications and patient trust, resulting in better overall experiences.”
The LG Patient Engagement Boards (ML5K-B Series) are designed to improve experiences for patients and caregivers while simplifying operations for administrators and IT staff. The 32-inch model can be rapidly deployed utilizing POE without the exhaustive approval process commonly associated with modifying a room’s electrical components. Convenience is further enhanced by each display’s automatic brightness sensor, which ensures viewing comfort by matching ambient light levels throughout the day.
Both models can be wall-mounted vertically or horizontally using 200×200 VESA mounts. And both displays feature LG IPS panels and offer up to 50,000 hours of life, making them ideal for 24/7 use. Integrated stereo speakers further simplify deployment and provide flexibility to host a variety of content.
The 43-inch 43ML5K-B offers a typical brightness of 500 nits and has a 25 percent haze treatment that reduces glare, while the 32-inch 32ML5K-B provides 400 nits through standard power, and 200 nits through PoE.
“From diagnostic displays and ultra-secure thin client computing solutions to these new Patient Engagement Boards, LG is dedicated to leveraging the latest display technologies to help hospitals and medical providers create enhanced spaces and deliver 21st century care,” Mottlau said. “As institutions look to design and build their hospital rooms of the future, LG will continue to innovate to meet their changing needs.”