For nearly 20 years, Bionix has supplied the healthcare industry with its innovative and dependable Lighted Ear Curettes, designed to illuminate and improve visualization of the ear canal for efficient and safe cerumen Removal.
Today, the next generation hits the market with the ClearLook Lighted Ear Curette. Bionix developed this generation by using valuable insight and feedback from medical professionals who regularly used the previous model of Lighted Ear Curettes.
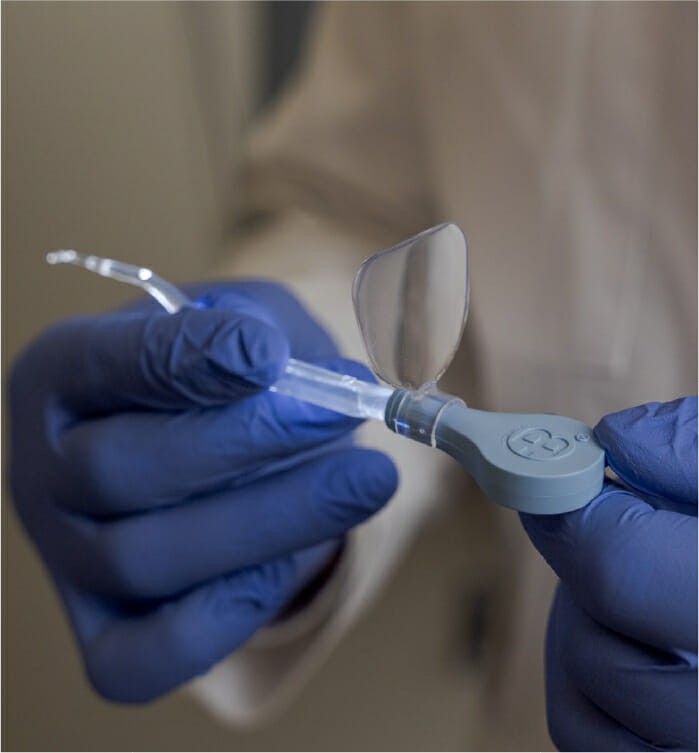
ClearLook Lighted Ear Curettes offer a unique and ergonomic handle design that promotes a natural and comfortable grip out of the line of sight, giving healthcare professionals better control and confidence while performing cerumen removal. ClearLook’s design includes reference markers for re-entry into the ear, improving practitioner and patient satisfaction.
Currently available in the popular FlexLoop® and VersaLoop® tip styles, ClearLook continues to employ illumination and magnification the same as the original Lighted Ear Curettes, but with a less obstructed, more accurate view of the ear canal.
“Bionix is pleased to offer healthcare practitioners this evolved version of our Lighted Ear Curettes that enhances the safety and time of cerumen removal procedures,” states Chris Becker, President and CEO, Bionix, LLC. “We greatly appreciate the ongoing feedback received from practitioners and clinicians as it allows our engineers to enrich our products and ensure that we continue to meet their needs.”
Bionix is committed to working with healthcare professionals to identify opportunities for innovation that enable advances in product development and deploy solutions that address challenges they encounter in patient care.
For a limited time, the new, just-released ClearLook Lighted Ear Curette are the same price as the current FlexLoop and VersaLoop Lighted Ear Curettes. Order now to take advantage of this introductory offer! The special offer expires December 31, 2022.