Limbitless Solutions develops life-changing prosthetic limbs for children using 3-D printing and sophisticated electronics which include Insight SiP’s RF modules for wireless communication enabling children to adjust their prosthetics through an app. Wearing these bionic arms can make the children feel like superheroes.
Limbitless Solutions is a non-profit organization created by a team from the University of Central Florida dedicated to using technology to improve children’s lives. It creates personalized and expressive bionic arms for children with limb differences, allowing the child to live a life without limits.
Advanced technology
The prosthetic limbs are made using 3-D printing and advanced electronics to create individual solutions for each child. Starting with several core designs, the child can customize the end result to suit his or her needs and personality. This technique keeps costs controllable, and the prosthetics affordable even for growing children, by orders of magnitude cheaper than conventional solutions.
Become a superhero
These Limbitless prosthetics dramatically improve the quality of life for their wearers. The prosthetics can be playful and fun, customized to make the children look and feel like a superhero.
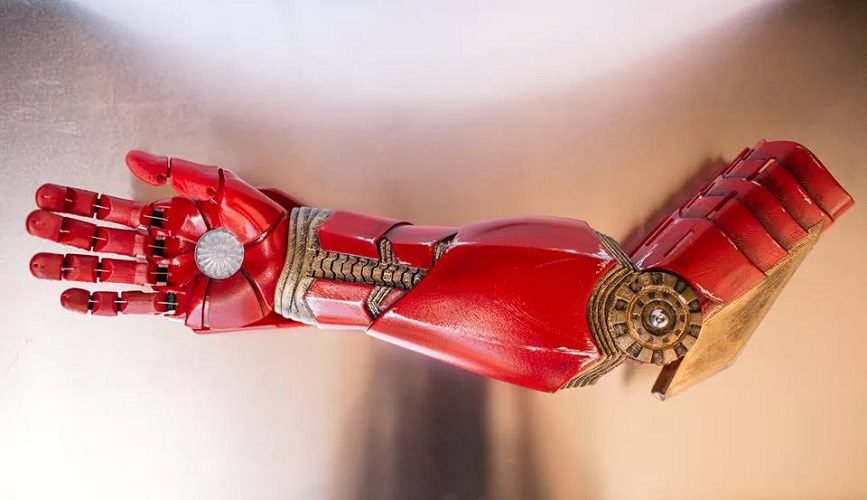
In one case, an “Iron Man” themed arm was presented to a child by the real-life Iron Man himself Robert Downey Jnr! (source: The Verge)
Sophisticated technology
To make the limb work, electromyographic sensors (EMG) pick up signals generated by muscle movements in the child’s arm. This is essentially the same technology as used in an electro-cardiogram (ECG) The sensors take the form of stickers placed on the upper arm. Below this is a socket to hold the prosthetic in place, matched to the individual child, and which fits regardless of whether or not the elbow is present. The forearm section of the bionic arm contains a battery pack that powers the motors controlling the different movements.
Hand unit
The hand unit contains the core electronics and motors for the fine control of the individual fingers. This unit includes the Insight SIP ISP1507-AX RF module and allows easy setup, adjustment, and monitoring of the arm. For instance, the degree of muscle-flexing required to generate a certain force in the fingers can be adjusted via an Android or IOS app, as well as provide easy access to status information such as battery level information. Even superheroes need to recharge from time to time!
Gamification
As well as the arm itself, Limbitless has developed games to assist the child in gaining control over their bionic arms. These are aimed at making the process of learning to use their new arms fun and engaging rather than potentially dull and frustrating. The Insight SIP module is also used in the game solution, to act as a wireless game controller for a computer or tablet-based game. These games take a classic level-based approach, starting with simple gestures such as opening and closing the hand and then moving on to more complex manipulations.
Solution choice
John Sparkman, Head of Research and Development at Limbitless Solutions said “We chose the Insight SIP module for its size and abilities. Our aim is to make our bionic arms as lightweight, comfortable, and easy to use as possible, and Insight SIP’s miniature BLE modules fit perfectly into this design philosophy.”
Nick Wood, Sales and Marketing Director at Insight SIP “We were delighted to hear about our products being used in such a wonderful solution. We are pleased that our technology has been able to contribute, even in a small way, to improving children’s lives.”
Albert Manero, Executive Director at Limbitless Solutions said “Kids with limb differences often face challenges. Our research team is focused on creating new robotics that makes everyday activities easier. Enabling parents to be able to easily calibrate and adjust the bionic arm via BLE made such a difference in their experience.