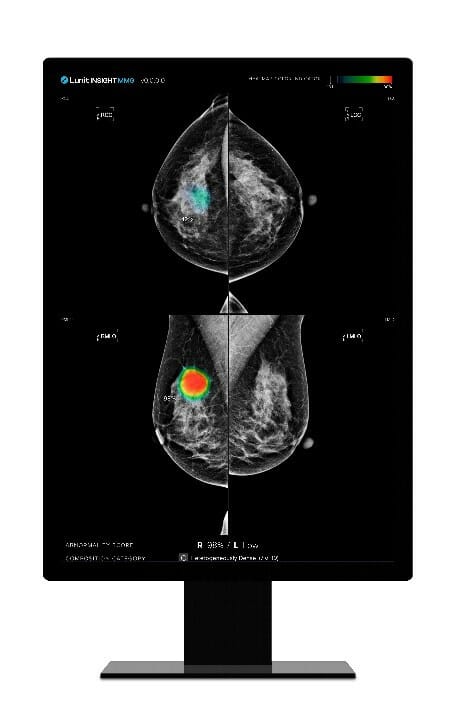
Lunit (KRX: 328130.KQ), a leading medical AI provider, today announced that it received commercial approval in Taiwan for Lunit INSIGHT MMG, the company’s AI solution for mammography analysis. Recently, the Taiwan Food and Drug Administration (TFDA) issued a class 2 medical device license for Lunit’s AI breast cancer detection solution.
Lunit INSIGHT MMG is one of the company’s most mature radiology products, analyzing mammography images with high speed and 96% accuracy. By detecting suspicious lesions in mammography images, the AI solution helps radiologists distinguish suspected tumor areas by providing the location of the lesion with an abnormality score reflecting the AI’s confidence level.
Following approvals by the U.S. FDA last year and Health Canada in June, Lunit’s commercial approval in Taiwan signals the company’s plans to actively expand in the Taiwanese medical device market.
Taiwan’s medical device market is growing steadily with the rising elderly population and the increasing import of high-tech medical devices. As of last year, the Taiwanese medical device market was NTD 77.785 billion and has grown by 3% annually since surpassing NTD 70 billion in 2019.[1]
“We started using Lunit INSIGHT MMG to speed up mammography interpretation and to reduce errors,” said Dr. Yeh Wei Cheng, director of the Department of Radiology, Nantou Hospital. “Lunit’s AI tool is particularly effective in helping diagnose dense breasts, which are often more likely to be misdiagnosed among East Asian women, and has helped improve communication between radiologists and breast surgeons.”
“Lunit INSIGHT MMG provides patients an opportunity to receive quick and accurate treatment while improving convenience for medical staff,” said Brandon Suh, CEO of Lunit. “We expect the approval to serve as a bridgehead to expand business across the greater China region.”
In July, Lunit announced that it signed a license agreement with Cathay Life Insurance, Taiwan’s largest insurance company, to officially integrate Lunit INSIGHT CXR, Lunit’s AI solution for chest x-ray, into Cathay Life’s current underwriting workflow.
[1] “Taiwanese Medical Devices Market Trend,” KOTRA (2022.08)