LifeSignals LX1550 Multiparameter Remote Monitoring Platform has received FDA Class II 510 (k) approval reports LifeSignals.
This follows recent CE marking and is further validation of LifeSignals’ drive to create innovative wireless platforms which can be used by clinicians for the continuous collection of patient physiological data at home and in healthcare settings.
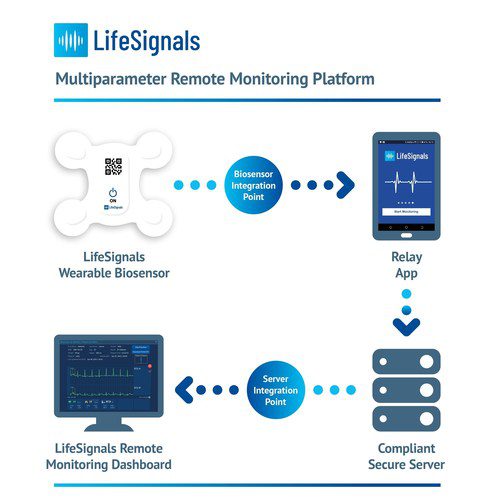
Central to the LX1550 Multiparameter Remote Monitoring Platform is a single-use wearable multiparameter biosensor that records Electrocardiography (2-channel ECG), Heart Rate, Respiration Rate, Skin Temperature and Body Posture data for up to five days. The encrypted physiological data can then be transmitted with high reliability from the biosensor, via a relay app to a secure cloud-based platform. Clinicians and Care Providers can access the cloud-based remote monitoring dashboard to view patient physiological data and manage alert settings.
The Remote Monitoring Platform is designed to enable healthtech companies to rapidly enhance their product and service portfolios to provide vital sign monitoring to the widest possible patient base, from any location. The relay app and dashboard are developer-friendly with ready-to-deploy software development kit APIs and is suitable for large scale implementation.
“COVID-19 has broken down the barriers to remote patient monitoring globally and I am proud of how the LifeSignals team has responded to develop the Platform in such a short space of time,” says Surendar Magar, Founder and CEO. “This FDA 510 (k) approval marks another major milestone in the company’s development and mission. It has already been implemented successfully in hospitals for monitoring COVID-19 patients in India and is being rolled out in Europe, UK, Singapore and the Philippines. Our focus now is to rapidly introduce low-cost remote vital sign monitoring to US healthtech companies looking to expand their services and improve patient care.”