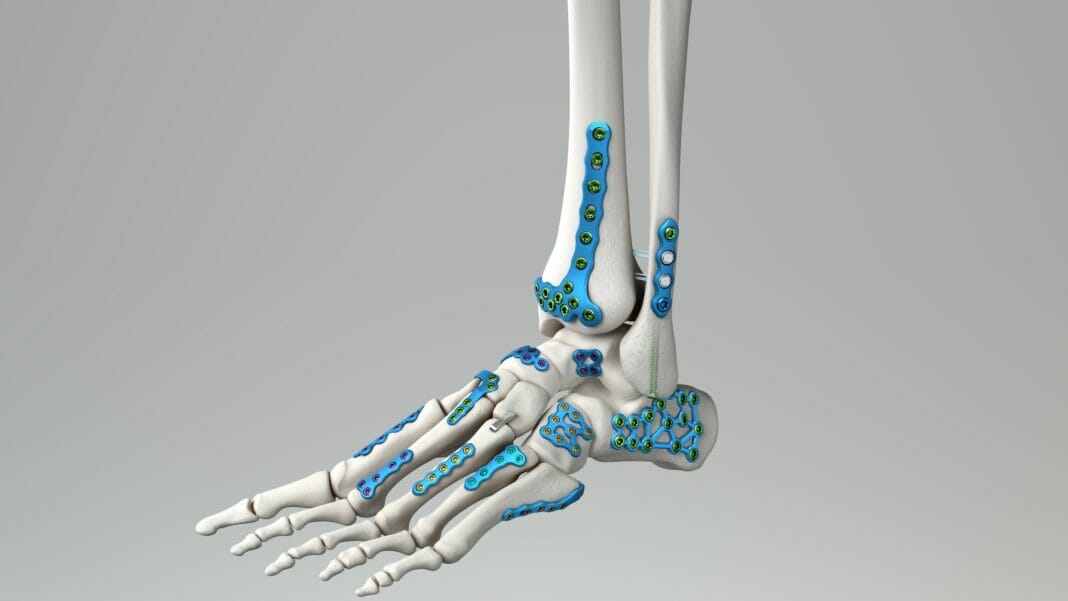
Medline UNITE Foot & Ankle announced today the launch of its FDA cleared Calcaneal Fracture Plating System and IM Fibula Implant.
Medline UNITE notes the national launch of these two products will provide surgeons with a comprehensive titanium foot and ankle trauma system to address nearly all fractures requiring ORIF with plate and screw fixation.
“Obtaining FDA clearance and launching these new implant systems comes on the heels of several other recent, successful launches, including the UNITE Jones Fracture Screw System, UNITE Distal Tibia Plating System, and SYNDEX™ with Constrictor® Technology Knotless Syndesmosis Implant System,” said Scott Goldstein, director of marketing at Medline UNITE Foot & Ankle. “Since Medline UNITE was first established in 2013, we have consistently and intentionally developed systems that offer the latest in implant design and technology, along with unique, bespoke instrumentation, and design rationale continuity across all systems. The result is a complete offering of implants that allows surgeons to treat patients and improve outcomes, with greater intraoperative speed and efficiency in the OR.”
Created around the philosophy of intelligent design, the Medline UNITE Calcaneal Fracture Plating System enables surgeons to handle a broad array of fracture patterns, surgical approaches, and anatomical variations from a single tray. The system is comprised of multiple implant options, including standard, offset, and extension Sinus Tarsi and Perimeter plates, as well as new, large fully threaded 5.5mm and 7.0mm headed cannulated screws. The system also comes equipped with a Sinus Tarsi Extension Plate Inserter, allowing for easy plate insertion and positioning through a sinus tarsi incision, enabling percutaneous screw placement in the posterior tuberosity.
The IM Fibula Implant joins the Medline UNITE Distal Tibia System as a standard option. The novel implant provides surgeons with a new option to treat patients with transverse fibula fractures requiring intramedullary fixation, often associated with distal tibia fractures.
“Unlike a traditional screw, the implant features a special tapered diameter designed specifically to fit within the fibula canal, and a dual-lead thread for faster insertion,” said Dr. Scott B. Shawen, MD of Charlotte, N.C. “It’s also available in lengths ranging from 65 – 150mm and comes standard in the tray, so our hospital and OR staff can avoid the excess cost and inefficiency of needing to locate and pull a separate tray with extra-long screws, such as a pelvic fracture set.”
“At Medline UNITE, we are guided by our design team, which is comprised of multiple fellowship-trained surgeons who bring with them decades of expertise and invaluable insights into the needs of their peers within the foot and ankle subspecialty,” Goldstein continued. “Through our collaboration, we have been able to identify crucial unmet needs and have challenged our engineering and product development teams to help advance clinical performance and improve surgical efficiency through intelligent design.”
Medline is a healthcare company; a manufacturer, distributor and solutions provider focused on improving the overall operating performance of healthcare. Medline works with both the country’s largest healthcare systems and independent facilities across the continuum of care to provide the clinical and supply chain resources required for long-term financial viability in delivering high quality care. With the size of one of the country’s largest companies and the agility of a family-owned business, Medline is able to invest in its customers for the long-term and rapidly respond with customized solutions. Headquartered in Northfield, Ill., Medline has 30,000+ employees worldwide, a fleet of nearly 1,200 trucks and does business in more than 125 countries.