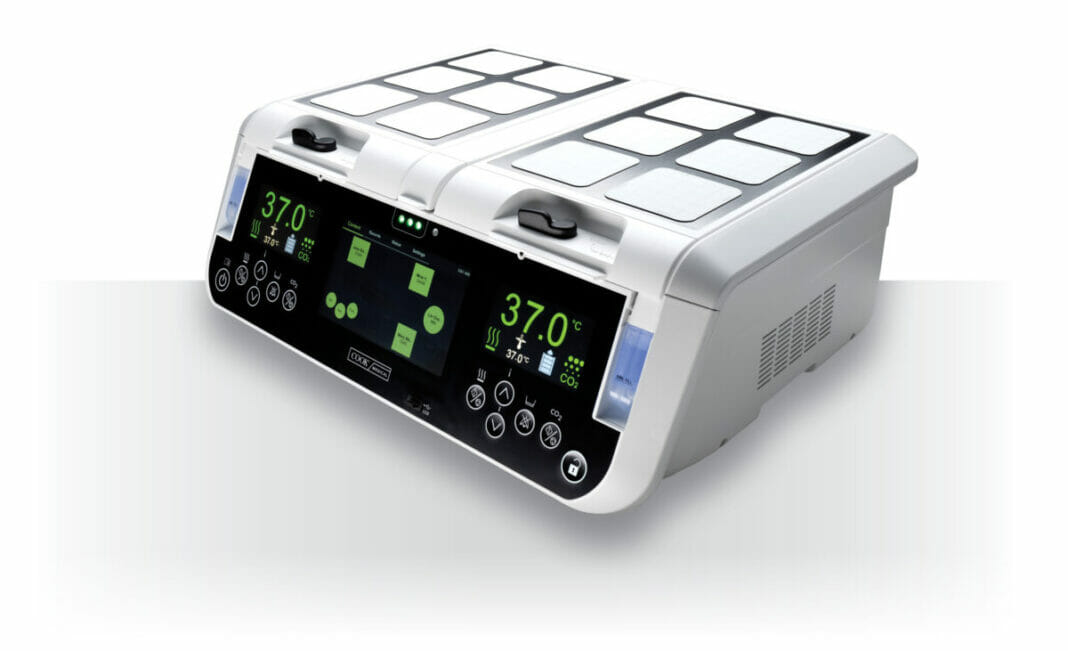
The MINC+ Benchtop Incubator is now available to clinical embryologists and IVF clinics in the US and Canada.
The MINC+ is the next generation of the MINC® Mini Incubator, a benchtop incubator that has been a standard component in IVF clinics for more than 20 years.
“We listened to user feedback when designing the MINC+ and incorporated new and updated features that will make a big difference for embryologists,” said Olin Emmanuel, global director of Cook Medical’s Reproductive Health specialty, “so we’re very excited to introduce this technology to the IVF world.”
The MINC+ incubator also includes the DishTrace platform, which combines functionality through the incubator touchscreen and a PC software program to provide a wide range of dish-data management tools.
With the DishTrace platform, users can access and log information via the touchscreen, track culture dishes as they are checked in and out of the incubator, monitor up to 50 MINC+ incubators, and generate and view graphical histories and reports of MINC+ activity.
The MINC+ Benchtop incubator is part of a suite of assisted reproductive technology (ART) products offered by Cook Medical, including culture media and devices for ovum aspiration, intrauterine insemination, and embryo transfer and manipulation.