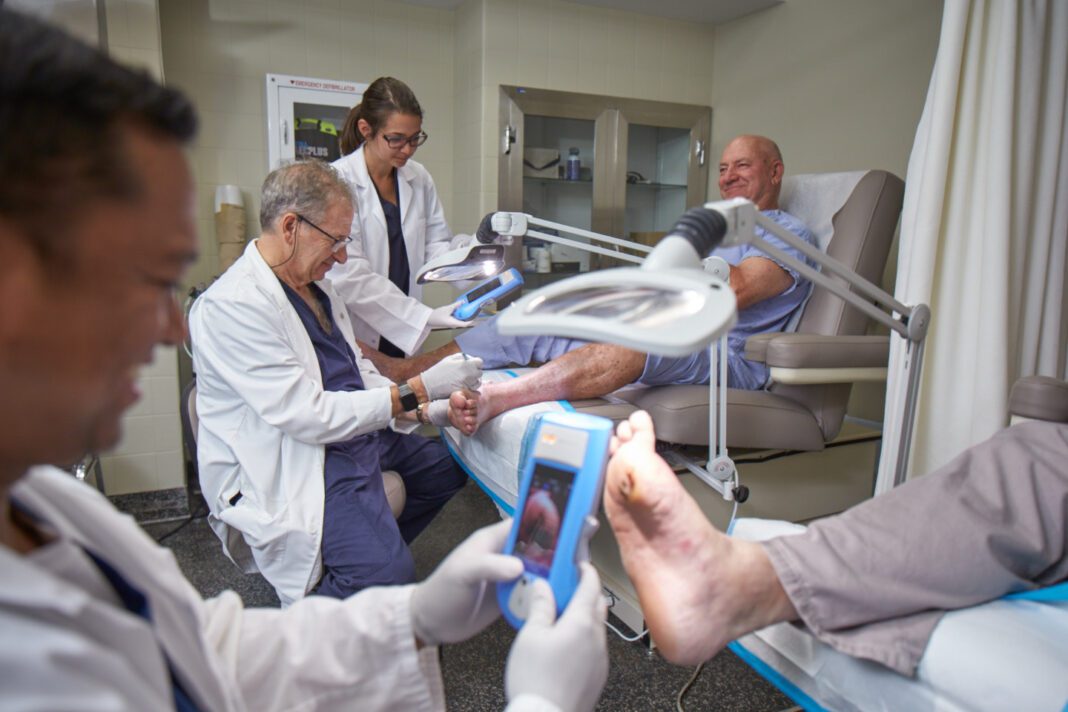
MolecuLight Inc. has announced that the MolecuLight i:X® device has successfully received regulatory clearance and is now commercially available to the wound care market in South Korea.
In addition, the MolecuLight device has also received reimbursement in Korea from the Ministry of Health and Welfare enabling clinician reimbursement for performing the medically necessary MolecuLight procedure.
Reimbursement for the MolecuLight procedure was granted by the Ministry of Health and Welfare of Korea, as per the notification number 259-858. This was announced based on Reimbursement data from the Korea New Medical Technology – Stability and Effectiveness Evaluation.
MolecuLight is exclusively distributed in South Korea by KOVE, Inc., a company specializing in providing novel products that assist in the treatment of diabetes foot ulcers in Korea including medical devices that assist with the diagnosis and treatment of wounds. KOVE’s team of clinical and technical support specialists have more than 30 years of experience in medical devices and wound care. KOVE also performs clinical research with many university hospitals in Korea.
The South Korean market for wound care is significant and can be understood through the pervasiveness of diabetes and diabetic foot ulcers. There are over 5 million Koreans with diabetes1, or 1 diabetic in every 30 adults. 25% of all diabetics also have a diabetic foot ulcer.
“The market for the MolecuLight device is significant in South Korea as there is a comprehensive and progressive health care system that quickly adopts new and clinically useful technologies”, says JUNHYOUNG LEE, CEO of KOVE, Inc. “Because of the high national insurance coverage for medical procedures, Korean patients readily visit hospitals to treat ailments and physicians are motivated to treat and monitor wounds until they are fully healed.
Lee added, “The MolecuLight technology provides real-time actionable information on wound bioburden and allows clinicians to make bedside decisions quickly. It will be well-received by the South Korean medical community. Significant demand for the MolecuLight device has been verified through market research conducted over the last 12 months as part of the registration and reimbursement process.”
“We are most impressed with KOVE, Inc., with their very experienced and responsive team and with their close relationship with the wound care community in South Korea“, says Anil Amlani, MolecuLight’s CEO. “We believe that the speed with which the MolecuLight i:X received both registration and reimbursement shows the quality of our clinical evidence and the proven clinical outcomes that clinicians can achieve using the MolecuLight device. We are confident that South Korea will become a major market for MolecuLight”.
MolecuLight’s broad body of clinical evidence includes 55+ peer-reviewed publications, including over 1,400 patients under study, showing the significant benefit of the MolecuLight i:X® to wound care clinicians in all care settings.