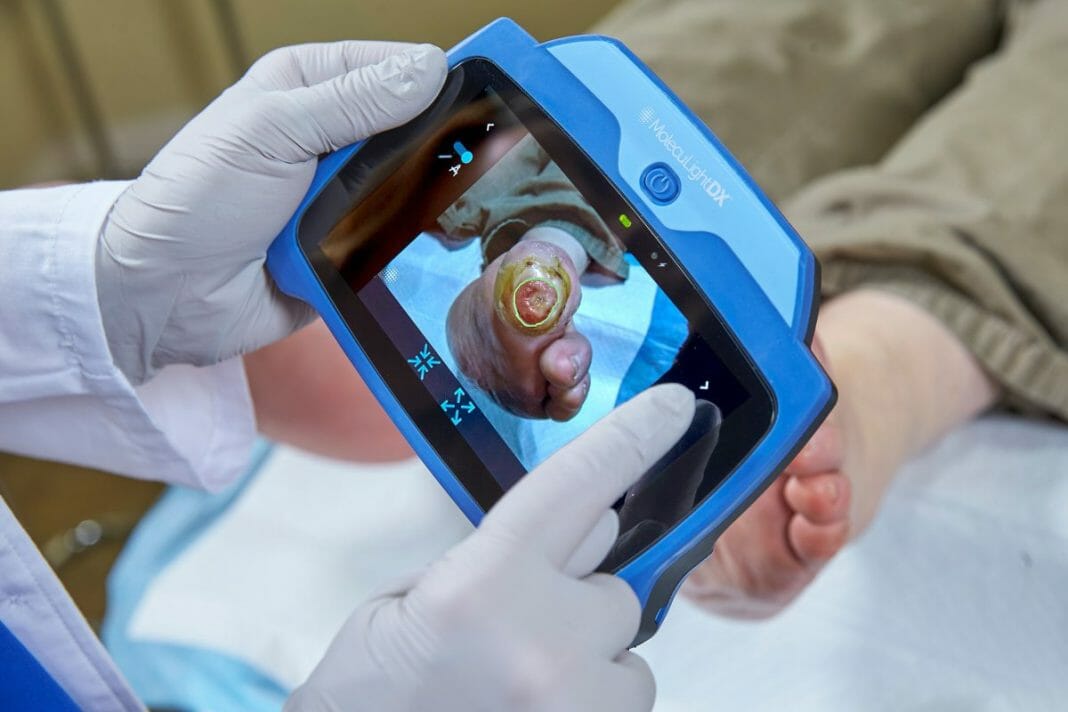
MolecuLight Corp., the leader in point-of-care fluorescence imaging for the real-time detection of bacteria in wounds, announces it has been awarded a new group purchasing agreement with AllSpire Health GPO, a Mid-Atlantic GPO and a partner of HealthTrust Purchasing Group, engaged with over fifty hospitals in Maryland, New Jersey and Pennsylvania.
AllSpire helps health systems optimize their operations by aggregating purchasing volumes, expenses, streamlining supplier negotiations and implementing efficiencies across the supply chain. The MolecuLight i:X® and DX™ wound imaging devices, which will now be available to AllSpire’s members, are helping clinicians to improve the state of wound care and ultimately to improve outcomes.
The MolecuLight imaging devices are the only FDA-approved devices that allow clinicians to visualize the presence, location, and load of bacteria (>104 CFU/g) in wounds in real-time. Published results from a recent 350-patient, 14-site clinical trial showed that the clinical standard of care alone detected 15% of wounds with elevated bacterial burden, while the addition of the MolecuLight device led to a 400% improvement in detecting these wounds2. The presence of elevated bacterial loads is known to impede wound healing1 and removal of bioburden is critical to improved wound outcomes1.
The i:X and DX provide invaluable bacterial information at the point-of-care to inform clinical decision-making and enable targeted wound therapies. In a 2022 randomized controlled trial (RCT) 3, the highest level of evidence-based research, the improvement in healing rate at 12 weeks doubled in the patients that had care informed by MolecuLight fluorescence imaging compared to those that were not. Improvements in the patients’ quality of life were also reported. Another recent study reporting increased wound healing rates with the incorporation of bacterial information from MolecuLight imaging also found substantially decreased use of antimicrobial dressings and systemic antibiotics4. The MolecuLight devices also perform accurate digital wound measurement, allowing for the consistent monitoring and documentation of wounds.
“We are thrilled to have entered into a supply contract with AllSpire Health GPO,” says Anil Amlani, MolecuLight’s CEO. “Through the i:X and DX, we hope to enable significant cost-savings and improvements in clinical outcomes. AllSpire’s extensive member base can now easily access the MolecuLight wound imaging devices and see the clinical benefits in their wound care practices.”
“We are most impressed with the clinical utility that the MolecuLight i:X and DX devices provide to wound care professionals and are pleased to offer the MolecuLight portfolio via our Group Purchasing Agreement to our member hospitals”, says James Wallick, Senior Director, Strategic Sourcing at AllSpire Health GPO. “AllSpire is dedicated to sourcing the most innovative products that help to improve clinical decision-making and cost-efficiencies. We believe that the MolecuLight devices are highly innovative and will help to provide such clinical and operational benefits”.
In addition to the clinical benefits, MolecuLight procedures performed in the United States can benefit from an available reimbursement pathway including two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” procedures and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment.
1 Caldwell et al. Surg Clin North Am, 2020, 100(4)
2 Le et al. Adv Wound Care, 2021
3 Rahma S. et.al Diabetes Care 2022;45(7):1601–1609
4 Price et al. Diagnostics, 2020