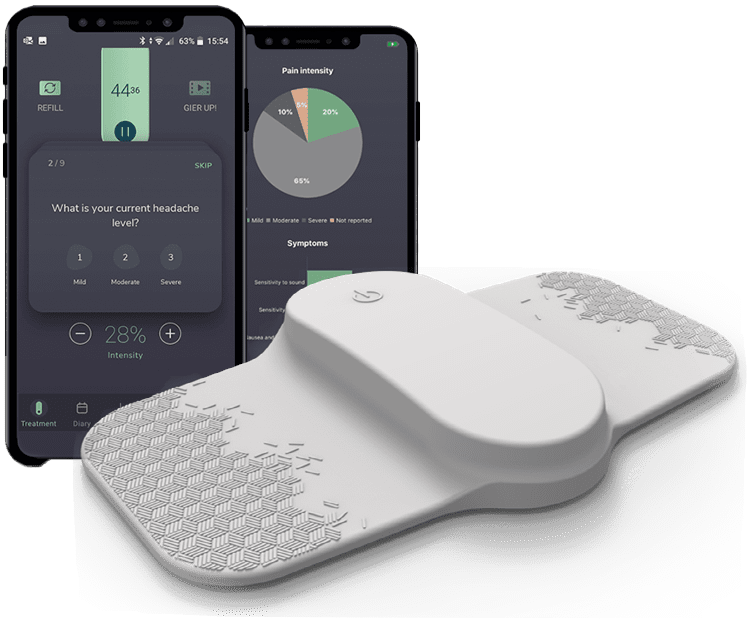
Nerivio has shown clinical efficacy for episodic and chronic migraine, both in clinical trials and real-world evidence analyses reports Jason Sico, MD, Associate Professor of Neurology and Internal Medicine in Yale School of Medicine.
He said, “Providing headache care to both Veterans and non-Veterans, it has been my experience that Veterans tend to live with migraine and related headache disorders at higher rates than the general population. Making available effective treatments is extremely important. Drug free options like Nerivio resonate with the values and preferences of many Veterans.”
A number of studies have outlined the prevalence of migraine amongst Veterans. 36% of Veterans who completed a 12-month deployment to Iraq exhibit symptoms of migraine – 3 times higher than the general population. Over nine million Veterans are enrolled in the VA health care system.
“As a US Navy veteran, I feel gratified to be able to provide Veterans who suffer from migraine access to Nerivio”, said Scott Szymanski, President Theranica US. “Veterans are one of the most at-risk populations for migraine and other headache disorders. We are committed to have Nerivio improve the quality of life of Veterans receiving medical care within the VA system, as well as those that are making the transition back to civilian life.”
“The VA is a role model for putting the well-being of its members at the highest priority,” said Alon Ironi, CEO and Co-founder of Theranica. “We are also in the process of negotiating agreements with several commercial health insurance organizations. A significant amount of clinical data has solidified that Nerivio® provides an effective and safe non-pharmacological alternative for treating migraine both in adults and adolescents, while also allowing patients and physicians to track the disease and understand how to best manage it through the Nerivio mobile application.”
Theranica’s FDA-cleared prescribed therapeutic wearable Nerivio® is worn on the upper arm upon onset of a migraine attack, and utilizes technology called Remote Electrical Neuromodulation to activate the brain’s native Conditioned Pain Modulation mechanism to treat pain and associated migraine symptoms. Nerivio was cited as one of TIME’s best inventions of 2019.