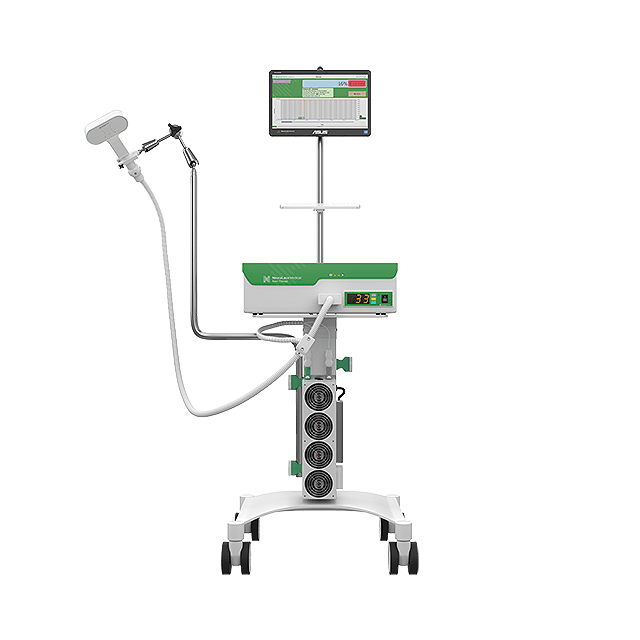
NeuraLace Medical, Inc., a medical device company focused on improving pain relief among patients suffering from debilitating chronic pain, announced today that it has received FDA 510(k) clearance for Axon Therapy® to non-invasively stimulate peripheral nerves and provide chronic nerve pain relief.
“After 10 years of R&D, we are pleased to have received FDA 510(k) clearance for Axon Therapy and we’re incredibly excited to bring to market a non-opioid treatment option that provides meaningful relief for patients suffering from chronic pain,” said Shiv Shukla, Founder and CEO of NeuraLace Medical.
He concluded, “We believe Axon Therapy is a significant step forward on the care continuum that is accessible to patients today and we look forward to bringing sustainable pain relief to patients by launching Axon Therapy in the United States.”
“Existing options to treat chronic nerve pain can be ineffective, addictive and invasive.” Said Executive Chairman, Sean Edwards. “We’re excited to offer patients a method of relief that is non-addictive and non-invasive.”