NeuroCatch (from HealthTech Connex Inc.) in Surrey, British Columbia and BIOFLEX (Meditech International Inc.) in Toronto, Ontario are pleased to announce an exciting new alliance that will advance research into the benefits of photobiomodulation therapy (PBMT) on brain health, including traumatic brain injury.
The two companies will work together to leverage their proprietary and innovative technologies, with the ultimate goal of providing health care professionals with new tools and techniques to enhance brain health and treat traumatic brain injury, including concussion.
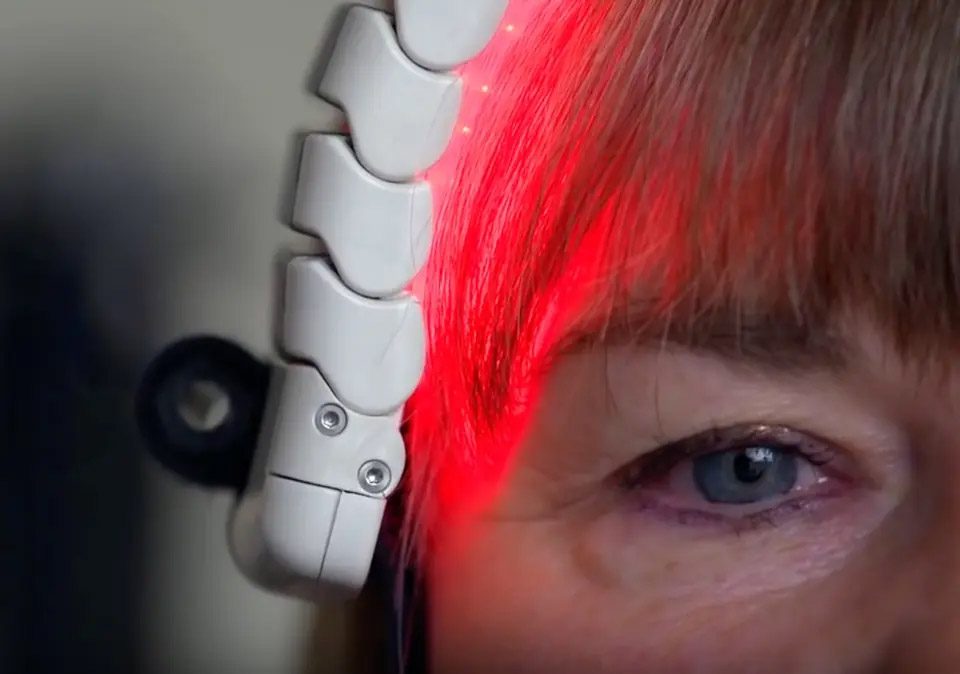
The partnership combines the rapid, objective, quantifiable insights into cognitive brain health from Platform with the growing exploration of BIOFLEX’s photobiomodulation technologies in the areas of brain health and traumatic brain injury.
“You can’t properly treat what you can’t measure,” says BIOFLEX CEO Nicholas Olteanu. “As BIOFLEX continues its mission to help people with traumatic brain injury, NeuroCatch can provide the objective cognitive assessments we require to gauge the effectiveness of PBMT on brain health and injury, and ultimately to help gain regulatory approval for the treatment of brain injuries. Our clinics have treated well over 6,000 TBI patients with highly positive results throughout our 33-year history, and we aim to extend our knowledge to our wide customer base through this collaboration.”
BIOFLEX ® PBMT utilizes scientifically developed and clinically tested protocols that maximize the effectiveness of treatment of an extensive range of clinical conditions. PBMT technology uses superluminous and laser diodes to treat diseased or traumatized tissue with photons. These particles of energy are selectively absorbed by photoacceptors in the cell and mitochondrial membranes initiating a cascade of complex physiological reactions, leading to the restoration of normal cell physiology. The exploration of photobiomodulation therapy on traumatic brain injury is a strong focus for the company, due to the prevalence and severity of this condition, and the current lack of effective therapies.
“NeuroCatch is excited to partner with BIOFLEX ® to advance the science of brain health,” says Kirk Fisher, CEO of NeuroCatch. “With recent breakthroughs that push the limits in sensitive detection of cognitive deficits, we believe that NeuroCatch can work synergistically with the BIOFLEX ® photobiomodulation platform to help measure the benefits of their technology as an innovative potential treatment for brain health.”