New Category III CPT Code for TherOx SSO2
ZOLL® Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today that the American Medical Association (AMA) CPT Editorial Panel issued a new Category III CPT® code for herOx SSO2, became effective July 1, 2021, for its TherOx® SuperSaturated Oxygen (SSO2) Therapy.
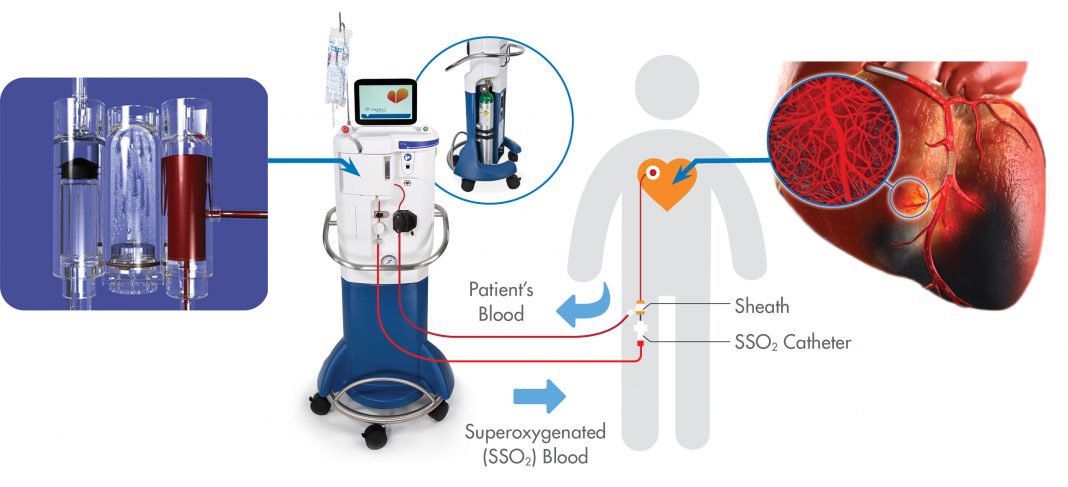
The code, 0659T (transcatheter intracoronary infusion of supersaturated oxygen in conjunction with percutaneous coronary revascularization during acute myocardial infarction, including catheter placement, imaging guidance [e.g., fluoroscopy], angiography, and radiologic supervision and interpretation), is effective July 1, 2021 for SSO2 Therapy including treatment of patients with left anterior descending ST-elevation myocardial infarction (LAD STEMI) heart attacks. SSO2 Therapy is the first and only FDA-approved treatment that has been clinically proven to significantly reduce cardiac muscle damage in heart attack patients after coronary angioplasty with stenting.1
SSO2 Therapy is indicated for patients who suffer LAD STEMI — also known as “widowmaker” heart attacks due to the high mortality rate — and are treated within six hours of symptom onset. The therapy delivers high levels of dissolved oxygen at 7–10 times the normal amount directly to the damaged heart muscle immediately after the coronary artery has been successfully opened via angioplasty and stenting. Multiple clinical trials have demonstrated the efficacy of SSO2 Therapy to reduce infarct size.1,2,3
The New Category III CPT Code for TherOx SSO2 Therapy was supported by the leadership of the Society for Cardiac Angiography and Interventions (SCAI) and the American College of Cardiology (ACC). The decision was based on a review of published, peer-reviewed literature, the need for an accurate national code for physicians to use in reporting SSO2, and the importance of SSO2 as an emerging, innovative technology.
“The AMA issuing a CPT code for TherOx’s SSO2 Therapy is a significant milestone for the millions of patients who could benefit from its use,” commented Neil Johnston, President of ZOLL Circulation. “More and more hospitals are adopting this therapy to treat patients with the most serious form of heart attacks, and now the Category III code provides another major step forward toward capturing data on SSO2 and demonstrating improved patient outcomes.”
CPT codes are granted by the AMA CPT Editorial Panel and are widely used by government payers, including Medicare and Medicaid, and commercial health plans to process claims and determine reimbursement for healthcare services and procedures.
SSO2 Therapy was developed by Irvine, California-based TherOx, Inc., now part of ZOLL Medical Corporation.
Other Zoll Medical news of interest here.