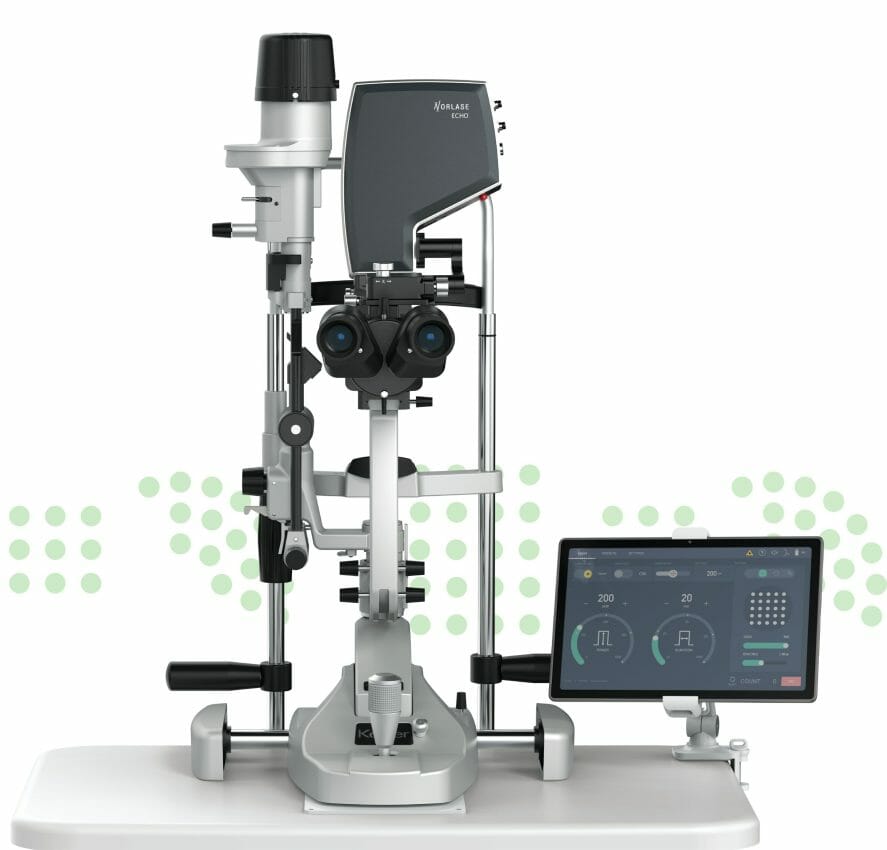
Norlase, a leading global ophthalmic laser manufacturer developing next-generation laser solutions, today announced it has received both FDA 510(k) clearance and CE Mark approval for the ECHO Green Pattern Laser photocoagulator. ECHO is an industry-first, portable scanning laser photocoagulator utilizing MEMS technology. The laser and scanner are completely integrated into a single delivery device that attaches to compatible slit lamps. Other exclusive features include no external fiber, a wireless user interface, µSec Mode for tissue-sparing treatment, and voice control of patterns and parameters. With an affordable price point, ECHO will make pattern scanning technology accessible to more doctors worldwide, equipping them with the tools to treat efficiently and increase patient throughput.
“The FDA clearance and CE mark approval of ECHO is our most significant milestone to date,” said Oliver Hvidt, CEO and Co-Founder of Norlase. “The launch of ECHO as the first and only truly portable pattern scanning laser brings a new paradigm to ophthalmologists that treat retina and glaucoma conditions. Our goal has been to develop a pattern laser that is accessible to all ophthalmologists, while maintaining our high level of innovation and precision. ECHO provides all of this in a smart, highly-intuitive product design enabling surgeons to treat with ease.”
Early adopters of the ECHO pattern laser have shared positive experiences about the laser’s functionality and treatment results.
“The ECHO laser is easy to use, intuitive and efficient,” said Dr. Firas Rahhal, Partner, Retina-Vitreous Associates Medical Group. “It provides excellent power and good uptake, even when used at lower power settings. The visualization on the slit lamp delivery is excellent, and in one of the cases I performed, this was true despite the presence of significant cataract. Due to these characteristics, along with others like the easy-to-use screen and controls, the ECHO is currently the best available slit lamp pattern laser that I’ve used.”
ECHO is the third product announcement since 2019, and is part of Norlase’s series of portable and affordable laser solutions, which are quickly modernizing the way doctors treat their patients.
ECHO Pattern Scanning Green Laser features include:
- Smallest physical footprint available in the industry
- Full pattern palette that includes grids, circle, and triple arc
- μSec Mode for tissue-sparing treatment
- Fiberless design to minimize costly service repairs and disruption
- Voice control of patterns and parameters
- Easily installed on any compatible slit-lamp


