January 27, 2021
Northwell Health reports The Feinstein Institutes for Medical Research and Cold Spring Harbor Laboratory (CSHL) are recruiting patients in a fully virtual, in-home, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of famotidine, or PEPCID, for the outpatient treatment of the novel coronavirus disease 2019 (COVID-19) in adults.
Sold over-the-counter as PEPCID, famotidine is a histamine 2 blocker used to decrease stomach acid and this clinical trial will assess its use as a COVID-19 therapy.
This is the first time an entirely virtual clinical trial has been initiated by CSHL and Northwell Health, New York’s largest health system, which has treated more than 100,000 COVID-19 patients since the start of the pandemic in March 2020. The trial is recruiting patients across the greater New York area who have tested positive for COVID-19, but are experiencing only mild to moderate symptoms that do not require hospitalization.
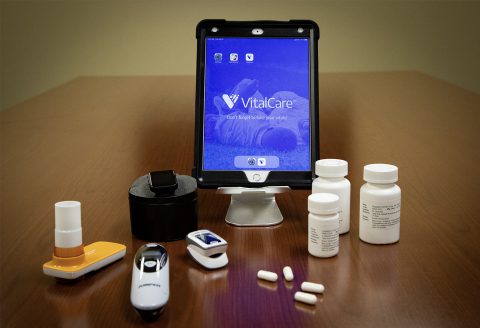
For those who qualify, and once consented, patients will be trained to use a cellular activated Apple iPad from their home, along with a Bluetooth enabled scale, thermometer, fitness tracker, spirometer (to study airflow in and out of the lungs), thermometer and pulse oximeter (to measure blood oxygen levels). Placebo or famotidine at 240 mg per day will be prescribed, and taken orally, for a maximum of 14 days. Northwell’s Home Lab program will be utilized for required blood draws and COVID-19 diagnostic nasal swabs tests. This trial is designed to keep trial participants completely out of the hospital throughout the course of their treatment.
“From the comfort of these patients’ own homes, we are taking the traditional clinical trial completely digital to study the efficacy and safety of a potential COVID-19 therapy,” said Tobias Janowitz, MD, PhD, a medical oncologist, principal investigator of the trial, assistant professor at CSHL, and adjunct professor at the Feinstein Institutes. “From assessment, to enrollment and daily data collection, we hope this study’s model will be an example for future clinical trials, and will provide high-quality data as we assess candidate treatments, like famotidine, to curb this disease.”
For those interested to learn more about the outpatient famotidine clinical trial, please email ClinicalTrials@northwell.edu.
Famotidine is a common, safe over-the-counter drug used to treat heartburn and assist with healing gastrointestinal ulcers. In April 2020, the Feinstein Institutes started the nation’s first famotidine clinical trial, which enrolled more than 230 patients. The initiation of the clinical trial was based on anecdotal reports from China that patients taking the drug had better outcomes from COVID-19.
“It’s been more than a year since this pandemic has spread across the globe, and there is only one fully-authorized COVID-19 therapeutic,” said Kevin J. Tracey, MD, president and CEO of the Feinstein Institutes. “This innovative clinical trial of patients in their own homes should provide important data to determine if a safe and inexpensive drug may be useful for COVID-19 patients.”
At the start of the pandemic the Feinstein Institutes established a COVID-19 Clinical Trial Unit (CCTU), a 200-member rapid-response clinical trial group of scientists, physicians, administrators and staff to review and launch clinical trials. Since March, the CCTU has enrolled patients in more than seven clinical trials and programs – including famotidine, remdesivir, sarilumab and convalescent plasma. Overall, more than 1,200 patients were enrolled into COVID-19 research trials across the health system, resulting in more than 200 manuscripts published, providing guidance to clinicians worldwide.
The outpatient famotidine trial has been funded by the Fast Grant and Pershing Square Foundation.