June 29, 2020
Olympus announces today the market availability of two single-use electrosurgical knives for Endoscopic Submucosal Dissection (ESD).
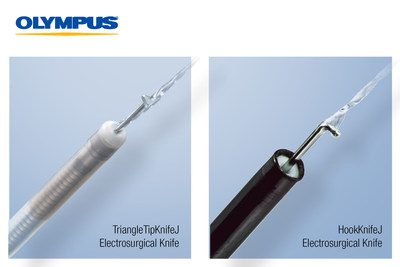
Olympus, a leader in ESD, is introducing to the U.S. the 510(k) cleared HookKnifeJ for the esophagus, stomach, and colon and the TriangleTipKnifeJ for the esophagus and stomach. The TriangleTipKnifeJ 510(k) clearance comes with a specific indication for Peroral Endoscopic Myotomy (POEM), a type of ESD used to perform esophageal myotomy for patients with swallowing disorders. Both knives feature integrated submucosal injection to support efficient, safe, and more reliable ESD performance and reduce the time required to perform an ESD procedure.
“We’re pleased to be able to launch the TriangleTipKnifeJ in time to finish out the June observance of swallowing disorders awareness month, as the TTJ is an important asset to POEM procedures, which can improve the quality of life for achalasia sufferers,” said Ross “Rusty” Segan MD, MBA, FACS, Global Chief Medical Officer at Olympus. “Quality of life improvements were behind the development of ESD for the lower GI tract as well, which is where the HookKnifeJ comes into play — ESD in ideal cases can allow for removal of certain cancers without the need for bowel resection and the potential need for a colostomy bag.
These two knives are some of the best examples of our ability to build on our global strengths in GI quality and innovation toward new approaches to treating disease.”
ESD is a technique of endoscopic resection that is being utilized in the United States for premalignant and early-stage malignant lesions of the GI tract. ESD offers an alternative to more invasive surgical procedures (e.g., colectomy, esophagectomy) and, especially for early cases, offers a promising alternative avoiding the costs and risks of surgery.
ESD has been shown to be appropriate for lesions that are 2 cm or greater in size and that have a flat, nonpendunculated shape. For these types of lesions, en bloc lesion removal can be accomplished with ESD. In this respect ESD is unlike endoscopic mucosal resection (EMR), which requires a piecemeal resection for lesions of this size and thus complete en bloc removal cannot typically be accomplished by EMR.i
Although the procedures are challenging and sometimes more time consuming, adoption in the U.S. is increasing, and more physicians are being trained on the associated skills, which include lesion marking via cautery, submucosal injection to lift the lesion, incision and precut of the mucosal layer, submucosal layer injection and dissection, and hemostasis during and after the procedure.
Innovative ESD knives provide physicians the precision instruments they need to conduct challenging procedures that minimize cutting through tissue, including the “tunneling” approach that uses separation of mucosal layers for scope egress, with the precision needed to avoid risk of damage to surrounding organs or blood vessels.
“Using the TriangleTipKnifeJ has added to the available armamentarium necessary in building our ESD practice, particularly our delivery of POEM procedures for the treatment of gastrointestinal motility disorders,” said Dr. Ryan Law, Assistant Professor of Medicine at the University of Michigan. “Submucosal tunneling is by nature a less invasive intervention when compared to traditional surgery. Patients tolerate these procedures remarkably well and have demonstrated high rates of technical and clinical success. The TriangleTipKnifeJ has been a positive addition to our practice.”
Bringing several design enhancements to ESD, the HookKnifeJ and TriangleTipKnifeJ knife benefits include:
- Clinical Efficiency: With the addition of a convenient jet, the new and evolved ESD knives help physicians work with more precision and reduce the time associated with replacing the device.
- Safety: Injection from the catheter instead of the needle tip allows for a small needle design, which is important to delicate areas of the anatomy.
- Reliability: The knives are designed to help take advantage of the hooking technique. Using this technique will help physicians dissect fibrotic tissue in a narrow lumen with precision and control — even from a perpendicular direction.
- Economic Value: Submucosal injection capability supports more reliable and more effective ESD performance.
Customers interested in the TriangleTipKnifeJ or the HookKnifeJ should contact their Olympus sales representative or Olympus customer service at 1-800-848-9024.