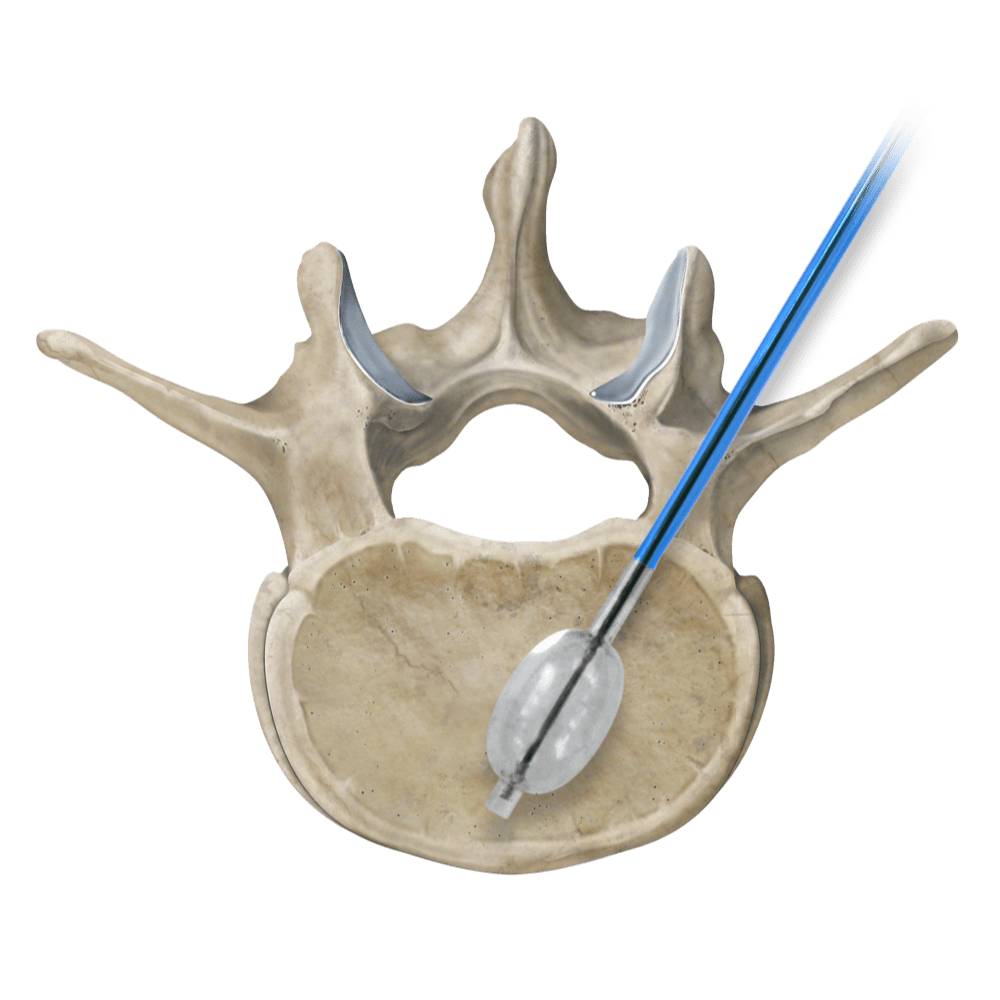
Osteo-Site Vertebral Balloon for vertebral augmentation has launched. The news was announced today by IZI Medical Products, a manufacturer of interventional radiology devices.
Osteo-Site Vertebral Balloon completes IZI’s offering of Vertebral Compression Fracture (“VCF”) treatment options, which include Osteo-Site Vertebroplasty, Osteo-Site Balloon Kyphoplasty, Blazer Curved Needle Augmentation, and Kiva PEEK Implant-based augmentation.
“We want to enable our physicians to choose the most clinically appropriate solution for their patients when treating vertebral compression fractures,” said Greg Groenke, CEO, IZI Medical. “We have created an integrated portfolio of VCF treatment devices with the ability to switch between different treatment options with ease.”
“IZI has completed the VCF toolbox: Kiva for metastatic VCF’s, Blazer Curved Needle for targeted cement deployment in sclerotic bone and blastic metastases, and now Osteo-site Balloon and curved needle for osteoporotic fractures. IZI has made it simple to switch between all of them which gives me intra-operative flexibility,” said Dr. Kieran Murphy, Professor of Radiology at University Health Network Toronto.
The new Osteo-site Vertebral Balloon is designed to create a cavity within the vertebral body. Currently, the balloon is available in 10G and 11G sizes. “The Osteo-site balloon will be paired with our Duro-Ject Injector, which provides maximum control and feedback during injection and minimizes radiation exposure with a long flexible tubing,” said Jovie Soriano, SVP Marketing and Business Development, IZI Medical. “We have also made improvements to our Murphy access needles based on physician feedback that will be included in the Osteo-Site Balloon kit.”
IZI Medical’s VCF portfolio will be supported by a dedicated sales team of direct representatives and independent distributors to drive market penetration in the United States, followed by expansion into Europe.